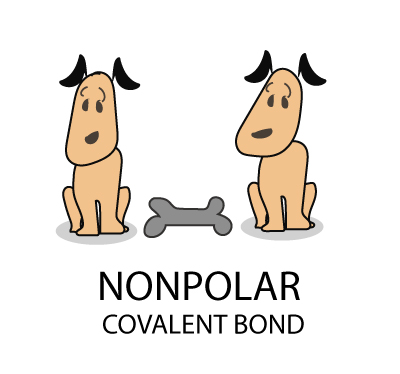
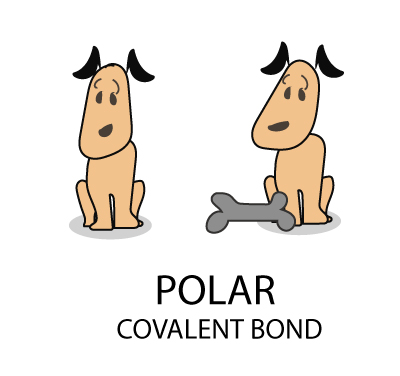
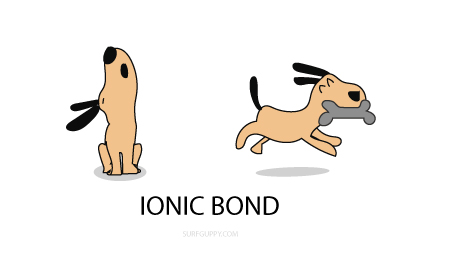
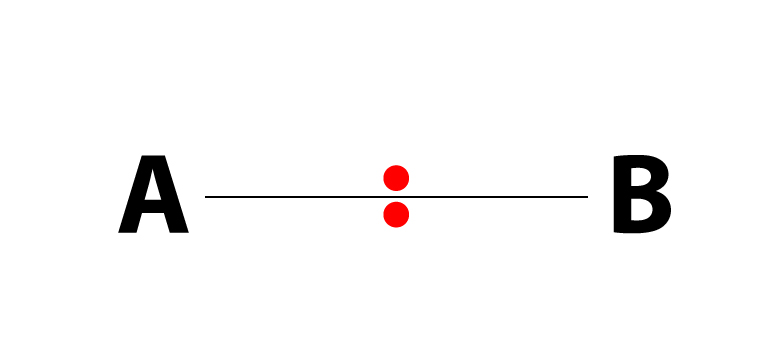
The Bohr Model
In 1913 Niels Bohr proposed a model to describe how energy levels are organized around an atom. While his model is now known to be incorrect, it is useful for understanding energy levels as Bohr’s model closely resembles planets orbiting Read More …