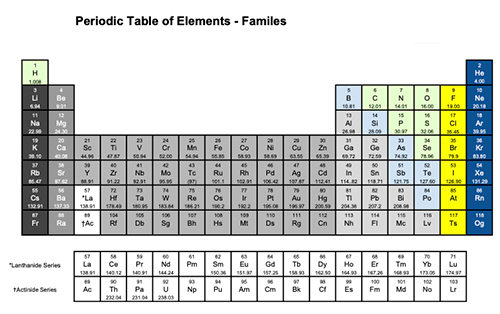
Today, we are going to learn about how the periodic table is organized and the important information that it provides.
Scientists discovered that if they arranged the elements according to their atomic number, properties of the elements would occur in a regular and repeating pattern.
This pattern is known as the periodic trends.
Terminologies
Before we delve into the periodic trends, let’s familiarize ourselves with a few new terms.
| Nuclear charge | The nuclear charge is equal to the number of protons in the nucleus of an atom. |
| Valence electrons | Valence electrons are electrons in the highest energy level and are furthest away from the nucleus |
| Core electrons | The inner electrons are called core electrons. Any electron that’s not a valence electron. |
| Bonding electrons | Bonding electrons are electrons involved in chemical bonding. Only valence electrons can be bonding electrons. |
Valence electrons
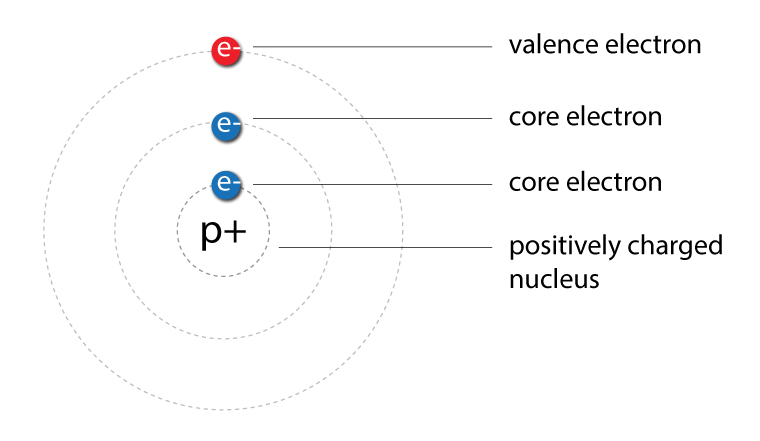 Valence electrons are those electrons found in the outermost shell of an atom.
Valence electrons are those electrons found in the outermost shell of an atom.
Electronegativity
When chemical reactions take place, atoms play tug of war with the valence electrons.
When two atoms form a bond, a pair of electrons is involved. These electrons are called bonding electrons.
Depending on which atom has a stronger pull, the pair of bonding electrons will move closer to that atom.
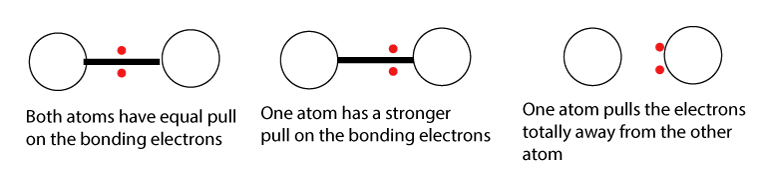
Electronegativity is a measure of an atom’s ability to attract a pair of bonding electrons. We will learn more about electronegativity in the electronegativity chapter.
Periodic trends
The major trends on the periodic table include:
- atomic radius
- electronegativity
- ionization energy>
- electron affinity
Atomic radius trend
Not all atoms are the same size. The size of an atom is dictated by how far the valence electrons are from the nucleus of the atom. An atom with a higher nuclear charge and lower number of shells tends to attract the valence electrons closer to itself.
- Across each row, the atomic number increases but the energy level does not change (electrons are all added to the same shell)
- The higher the atomic number, the higher the nuclear charge
- More nuclear charge means the electrons are pulled closer to the nucleus, and hence reducing the atomic radius
The atomic radius of atoms generally decreases
from left to right across a period.
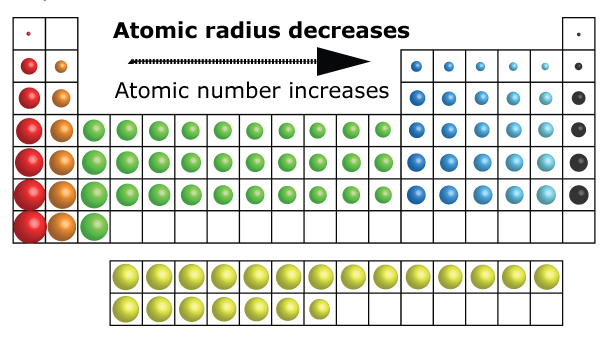
Down a group
Going down the group, a different trend unfolds. As the atomic number increases, the atomic radius also increases.
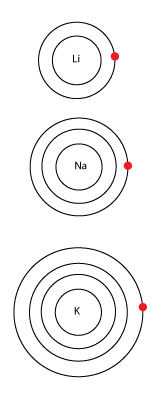
Going down a group, although the number of protons increases, the number of core electrons also increases.
At the end of each row (at the noble gases), the outermost shell has been filled and a new outermost shell begins at the start of the next row.
Electron repulsion starts to become significant as electrons in lower shells push away electrons in higher shells.
Due to the electron repulsion, the attractive forces of the nucleus is reduced. This is called the “shielding” effect.
“Effective nuclear charge” is a term used to describe the reduced charge felt by an electron due to shielding.
Potassium (K) vs lithium (Li)
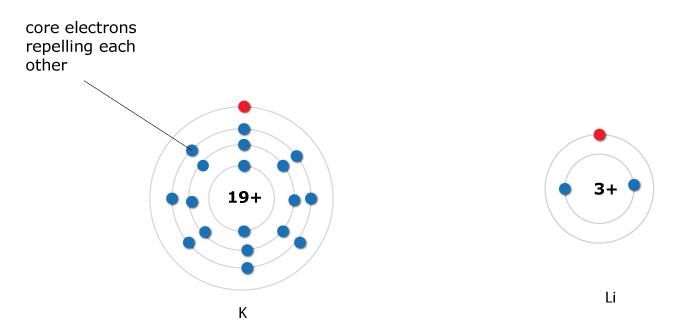
- K has more energy levels than Li
- Due to the presence of several layers of electrons, the attraction between the outer electron and the nucleus of the atom is reduced
- Hence, the valence electron for the K atom is less tightly held
- So, we expect the atomic radius of K to be larger


very helpful information for me.
what is difference between energy levels and ionization energy ? is it true LI has more ionization than K ?
SO why k has more energy level than LI ?
Ionization energy is the minimum energy required to remove one electron from the atom or ion in its ground state.
Potassium has a lower first ionization energy than lithium.