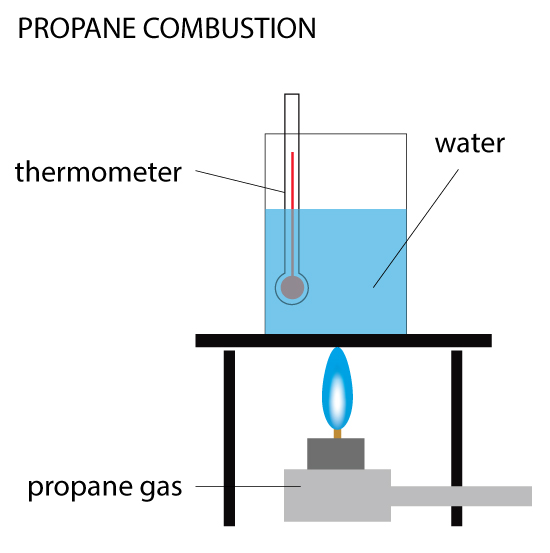
Open, Closed & Isolated Systems
Open, Closed & Isolated Systems A system refers to any parts of the universe being studied. If you are conducting an experiment in a beaker, then the system you are studying is in the beaker. The system is subject to Read More …