
(Picture above – dog escaping over the fence – winter 2015)
A spontaneous process
In life, many things happen spontaneously. For example, my dog escaped over the fence in the winter. It was spontaneous as she just walked over the fence on her own accord with no real special effort. The condition was just right as the snow was piled up so high that she could do that.
Everyday, there are many chemical reactions that take place spontaneously, but aren’t as exciting as my dog escaping. One example is rusting. It occurs on its own without the need of a continuous supply of energy. However, the right conditions must exist first to allow it to happen.
A non-spontaneous process
An example of a non-spontaneous process is pumping water uphill. Water will naturally flow downhill but for water to flow uphill, a pump is needed which requires energy to run it.
Exothermic and endothermic processes
When a spontaneous reaction takes place, it either gives off or absorbs heat. In rusting, the process gives off heat.
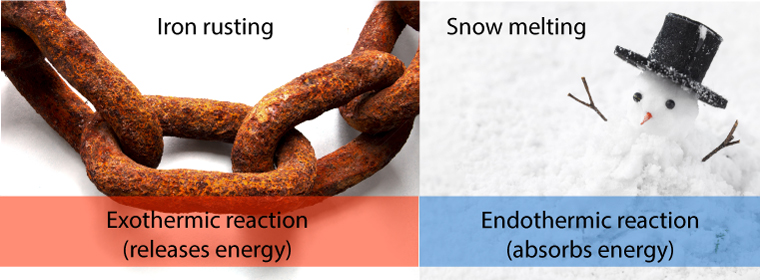
Iron rusting is a spontaneous process – it occurs naturally – but it can take a long time to happen.
Snow melting is a spontaneous process – it occurs naturally – it can occur very quickly if the weather is warm – or slowly if the weather is just above freezing.
| Process | Spontaneity | Speed | Heat exchange |
| Rusting | spontaneous | slowly | exothermic |
| Snow melting | spontaneous | can be quickly or slowly | endothermic |
Concept check
| Process | Spontaneous or non-spontaneous?
Exothermic or endothermic? |
| A combustion reaction | (Spontaneous & exothermic)
Combustion gives off heat and once the fire is started, it proceeds naturally. |
| Ice cubes melting at -1ºC | (Non-spontaneous & endothermic)
A continuous input of heat is required to melt ice cubes below freezing temperatures |
| Dissolving salt in water. | (Spontaneous & endothermic)
Salt will dissolve in water naturally without any intervention. The temperature dropping in the water indicates an endothermic process as heat is absorbed from the water. |
| Baking a cake | (Non-spontaneous & endothermic)
Baking requires a high temperature in the oven to cook the ingredients. |
| Photosynthesis | (Non-spontaneous & endothermic)
Photosynthesis requires energy in form of light to occur. At night photosynthesis stops because there is no light. |
Summary
A spontaneous process can be exothermic or endothermic.
A spontaneous process can occur quickly or slowly.
This will lead to your study of the next chapter ENTROPY.

