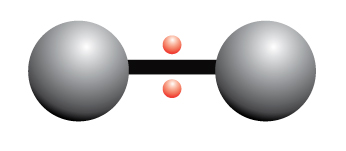
A nonpolar covalent bond is a chemical bond where electrons are shared equally between two atoms
The electrons are shared equally between the two atoms
Nonpolar covalent bond electronegativity scale
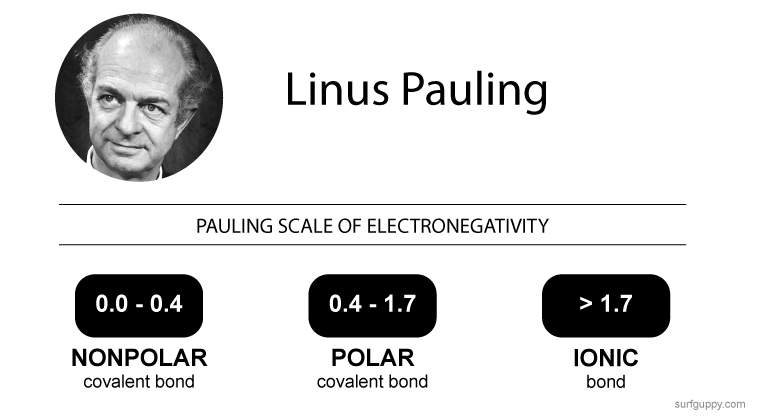
If the difference in electronegativity between two atoms is 0.4 or less, the bond formed between the two atoms is a nonpolar covalent bond.
Examples of nonpolar bond
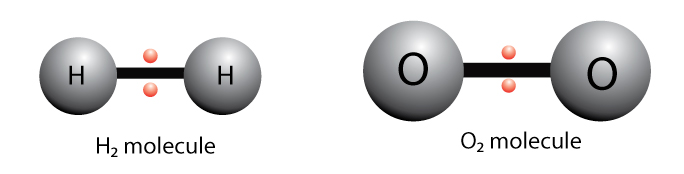
Nonpolar covalent bonds tend to form between two very similar atoms.
For example, two hydrogen atoms bond covalently to form an H2 molecule or two oxygen atoms bond covalently to form an O2 molecule.
An example of nonpolar covalent bond is a bond between two carbon atoms.
To read more about this bond type, go to Carbon to carbon nonpolar covalent bond>


This is really helpful! Thanks!