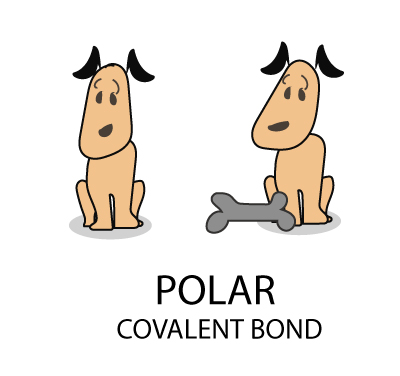
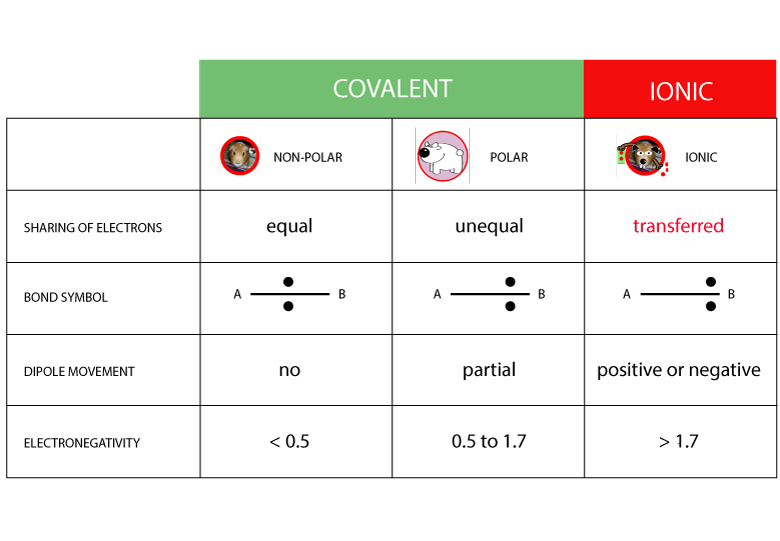
Nonpolar Covalent Bond
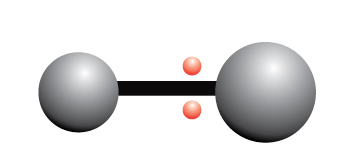
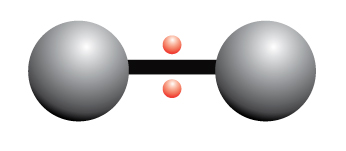
A nonpolar covalent bond is a chemical bond where electrons are shared equally between two atoms
The electrons are shared equally between the two atoms
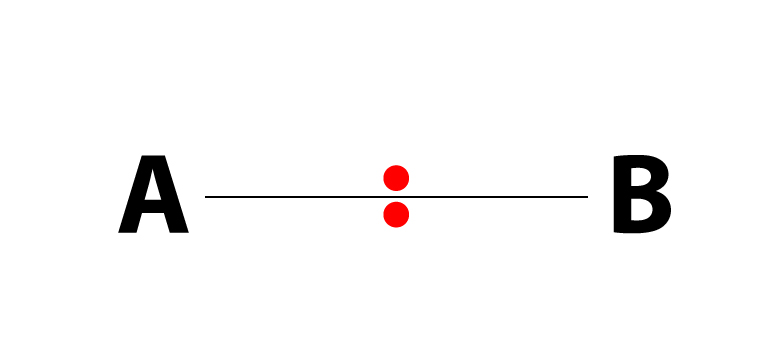
Nonpolar covalent bond electronegativity scale
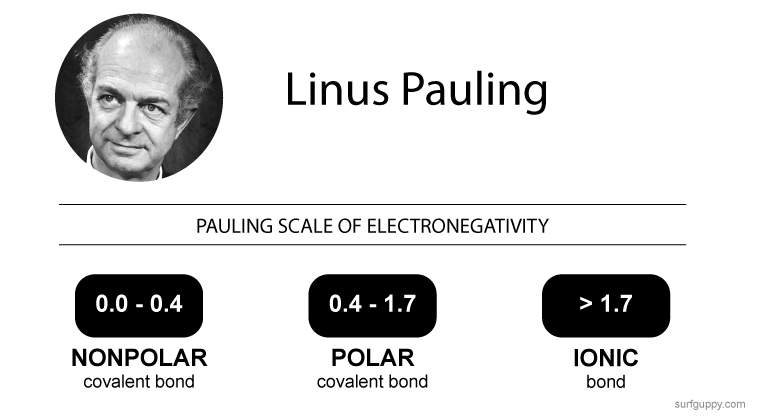
If the difference in electronegativity between two atoms is 0.4 or less, the bond formed between the two atoms is a nonpolar covalent bond.… Read the rest