What is a COVALENT BOND?
The term covalent bond is used to describe the bonds in compounds that result from the sharing of one or more pairs of electrons.
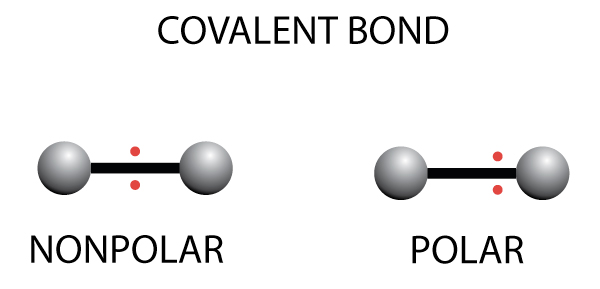
- There are two types of covalent bonds – nonpolar and polar covalent bonds
- In nonpolar covalent bonds, the electrons are shared equally between the two atoms
- In polar covalent bonds, the electrons are are not shared equally and will be closer to the atom with the higher electronegativity
- Please note: The electrons are shared, not transferred so there is no loss or gain of electrons