Freezing Point Depression

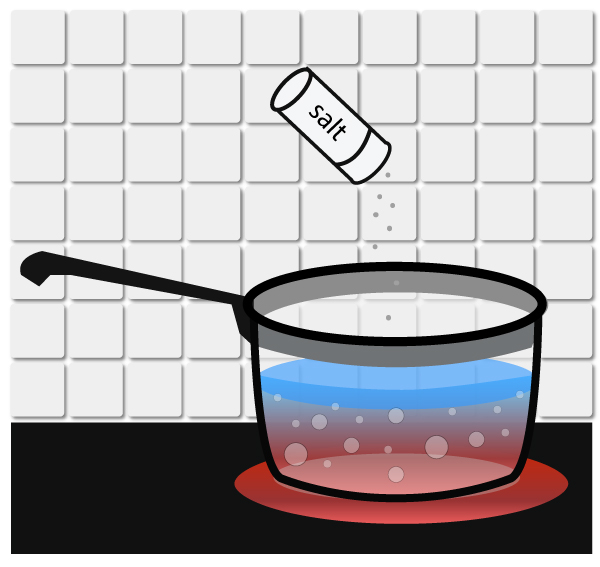
Freezing point depression is simply the process of LOWERING THE FREEZING POINT OF A LIQUID by adding a solute to it.
Ordinarily, water freezes at 32°F (0°C), but can you add salt to lower its freezing point to 20°F (-6°C). That’s why we use salt to melt ice on the road in the winter!… Read the rest