
Freezing point depression is simply the process of LOWERING THE FREEZING POINT OF A LIQUID by adding a solute to it.
Ordinarily, water freezes at 32°F (0°C), but can you add salt to lower its freezing point to 20°F (-6°C). That’s why we use salt to melt ice on the road in the winter!
Lowering the freezing point of water
During freezing, the water molecules instead of being able to move around randomly settle to a more organized structure called the solid state. Ice is the solid state of water.
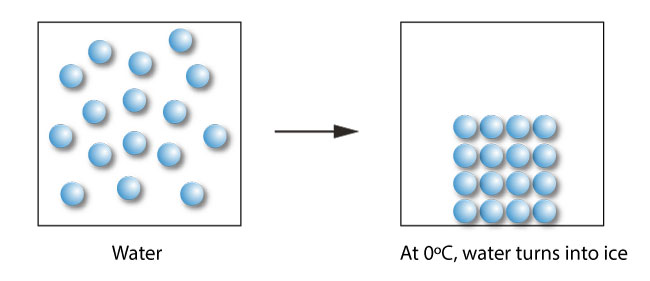
By adding salt (a solute) to water (a solvent), the freezing point of the solution (solvent + solute) is lower than that of a pure water.
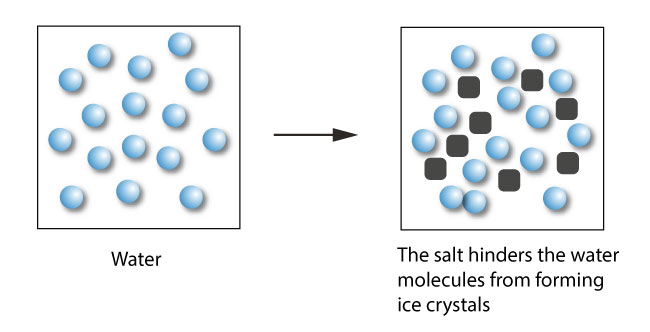
Freezing-point depression describes the process in which adding a solute to a solvent decreases the freezing point of the solvent.
Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities in a finely powdered drug. In the last case, the added compound is the solute, and the original solid is thought of as the solvent. The resulting solution or solid-solid mixture has a lower freezing point than the pure solvent or solid. This phenomenon is what causes sea water, a mixture of salt and other things in water, to remain liquid at temperatures below 0 °C (32 °F), the freezing point of pure water.
Colligative properties
Freezing point depression is a colligative property, which is a property of solutions that is affected by the number of particles in a volume of solvent (the concentration) and not on the identity of the solute particles (the type of solutes).
Real world applications

Ice cream freezes at a lower temperature than water because the sugar and fat in the mixture hinders the formation of ice crystals. It takes a colder temperature to get the water in the ice cream to freeze. The freezing point depression is an important phenomenal in ice-cream making!

So to make ice creams, you would need to cool the mixture down to about -5ºC, which is much lower than the freezing temperature of water.

