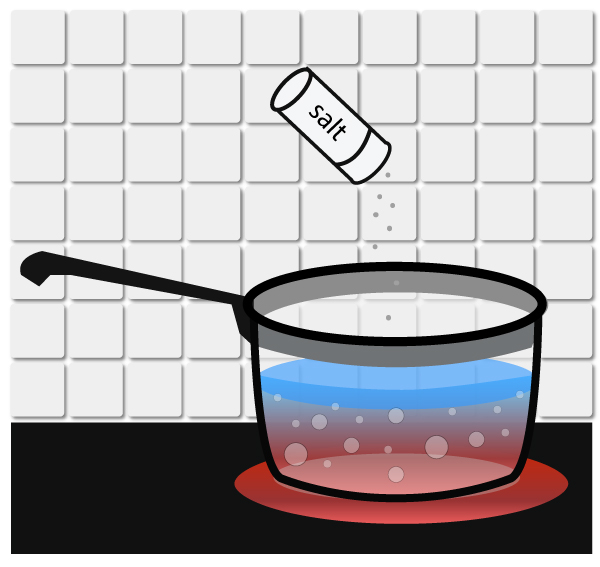
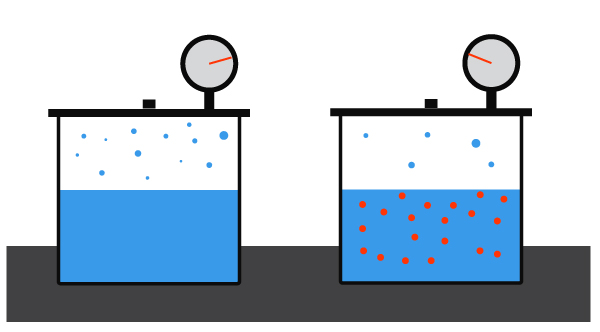
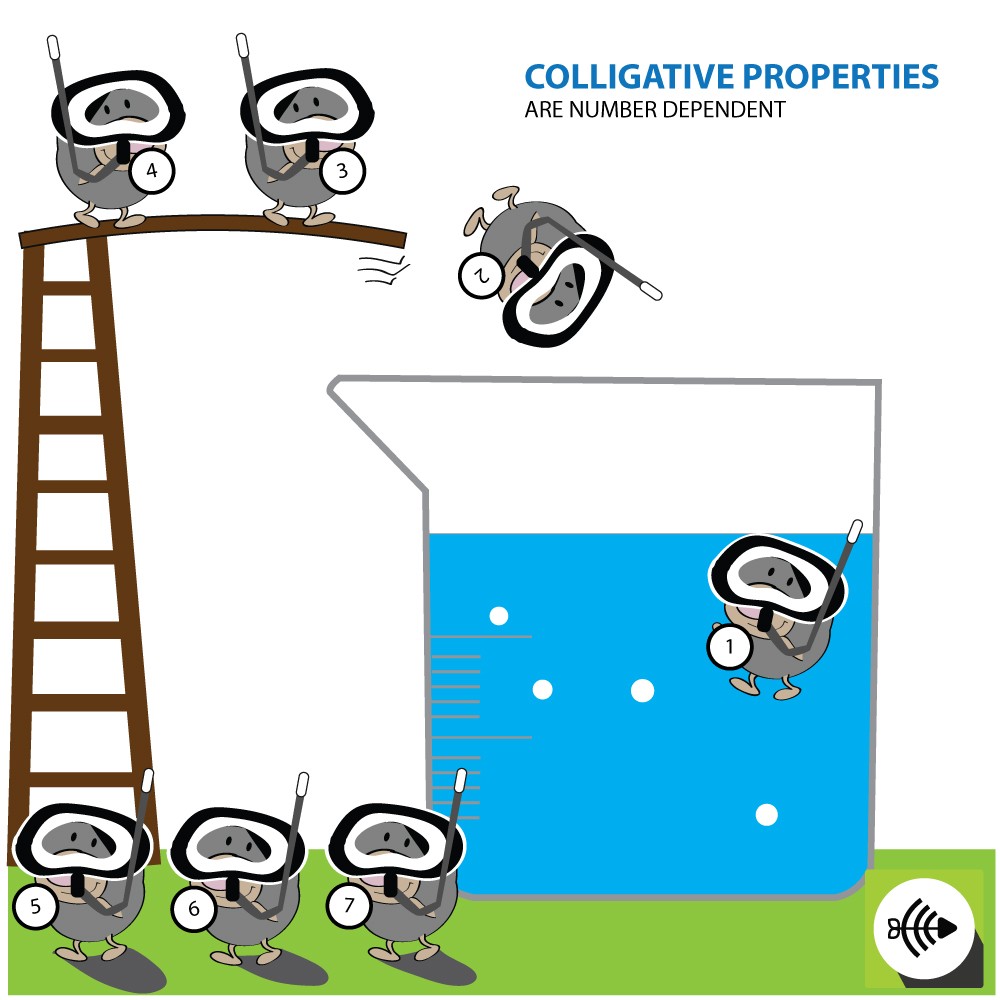
Atmospheric Pressure & Boiling Point
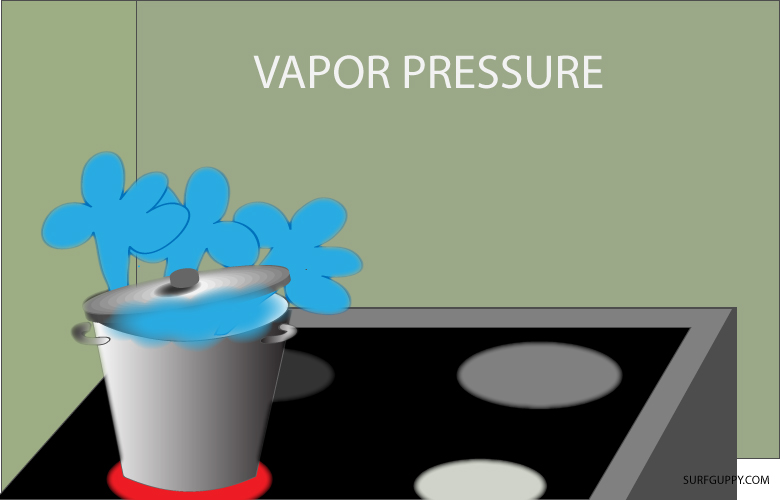
Water always evaporates no matter what the temperature or atmospheric pressure is. Even in the coldest of winter, snow and ice can evaporate by a process called sublimation. They can turn straight into vapor (the gaseous stage) without melting. The Read More …