An ionic bond
An ionic bond is formed between elements of large differences in electronegativities, which are typically found between metals and non-metals.
The atom that loses the electron(s) becomes a positively charged ion called a cation, and the one that gains the electron(s) is called the anion.
Electronegativity bond scale
For ionic bonds, the electronegativity (EN) difference between two atoms is greater than 1.7
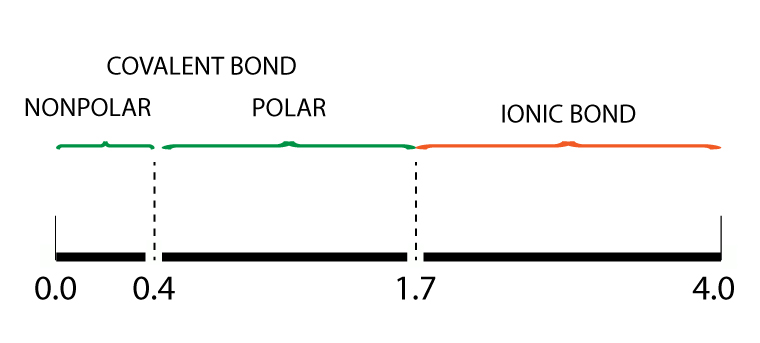
The chart above shows the relationship between electronegativity difference and the bond type.
Electronegativity difference between Na and Cl
Let’s look at an example of an ionic bond formed between a sodium (Na) and chlorine (Cl) atom.
The electronegativity difference between sodium and chlorine is shown below:
| Element | Electronegativity |
| Sodium, Na | 0.93 |
| Chlorine. Cl | 3.16 |
| EN Difference = (3.16 – 0.93)
= 2.23 |
Since the difference is greater than 1.7, the bond between sodium and chlorine is considered an ionic bond.
Sodium chloride, NaCl
Sodium (Na) has 1 valence electron
Chlorine (Cl) has 7 valence electrons
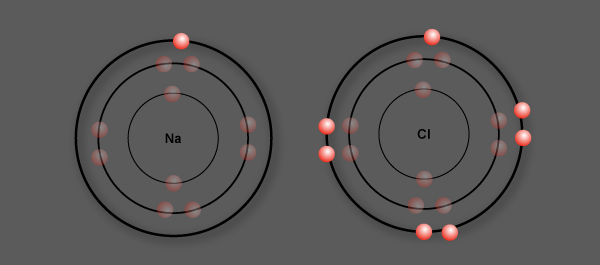
Chlorine is more electronegative than sodium
Sodium loses its valence electron to chlorine
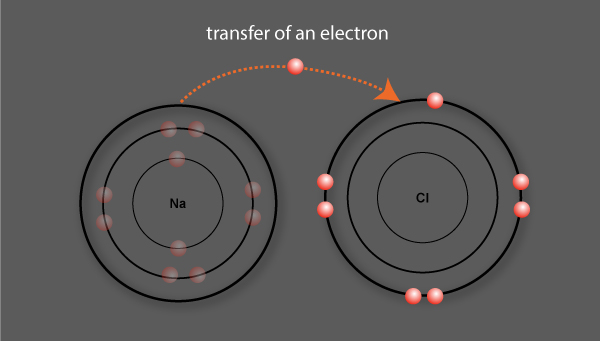
An ionic bond is formed between sodium and chlorine
An electron is transferred in the formation of the ionic bond
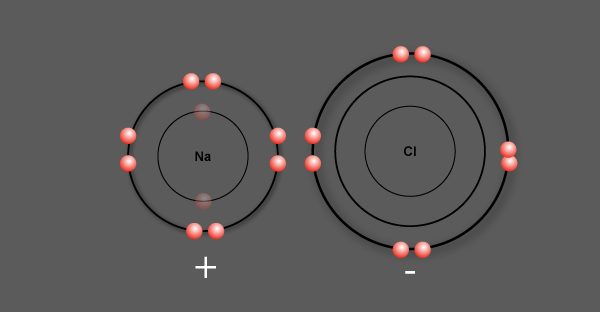
Sodium, having lost 1 electron, becomes a positively charged ion called a cation
Chlorine, having gained 1 electron, becomes a negatively charged ion called an anion
Now, these oppositely charged ions attract each other

