
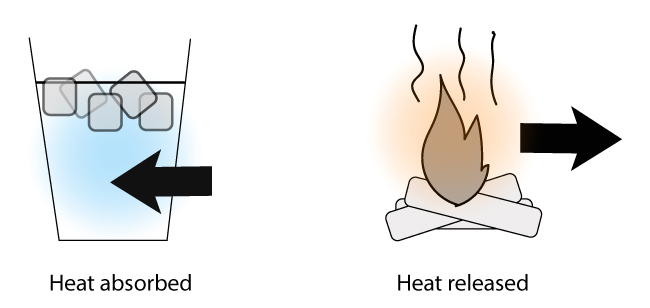
The term enthalpy change is a term to describe the amount of heat that passes in or out of a system during a chemical reaction or a physical process.
- You: How much energy is absorbed when the ice melts in the cup?
- Science: What’s the enthalpy change when the ice melts in the cup?
- You: How much energy is released when the logs burns?
- Science: What’s the enthalpy change when the logs burns?
Calorimeter
In school, you can measure the heat exchange of a reaction in a device called a calorimeter. The experiment is conducted under atmospheric pressure which is constant. By measuring the temperature rise or drop for the reaction, you can determine the heat exchange – or the enthalpy change – of the system with its surrounding.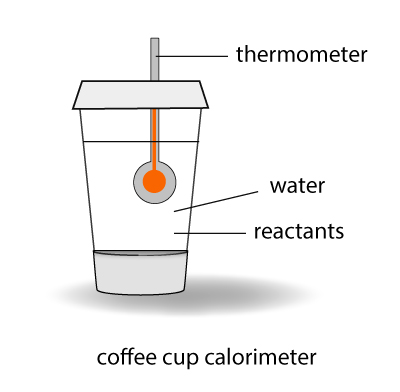
Using the calorimeter in school isn’t the most accurate way of determining enthalpy change of a reaction because of heat losses. However, the experiment can be conducted easily in the laboratory and it will give a good approximation for the enthalpy value by assuming heat is not lost to the surroundings.
Delta H
Enthalpy change is expressed as:
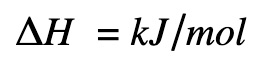
kJ = kiloJoules
mol = moles
A combustion reaction
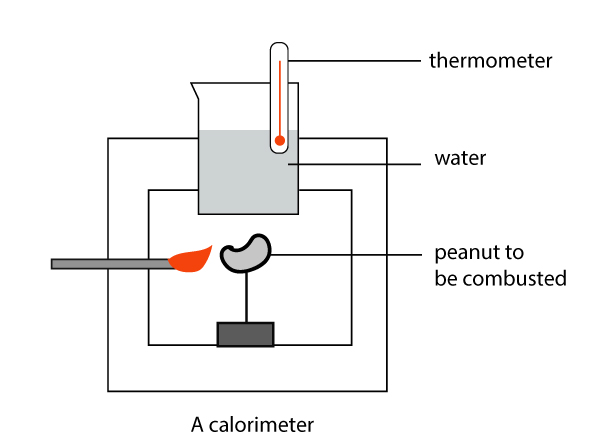
Calorimeters come in many shapes and sizes. The above shows an example of an experiment to measure the enthalpy change in a combustion reaction (eg. burning peanuts). In a combustion reaction, the substance reacts with oxygen in the air to give off energy in the form of light and heat.
In the food industry, food calorimetry is a technique used in measuring the calories of food. Expensive calorimeters are used to conduct combustion reactions to determine the amount of energy in the food.
Endothermic & exothermic
- If the enthalpy change is n e g a t i v e, the reaction is exothermic — it releases heat.
- If enthalpy change is p o s i t i v e, the reaction is endothermic — it absorbs heat.
Sodium chloride and hydrochloric acid reaction
Sodium and chloride combines to become sodium chloride (table salt)
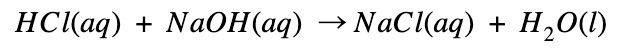
- During this process, heat is released
- The energy of the product (NaCl) is lower than that of the reactants (Na + Cl).
- Therefore the enthalpy change is negative
Is photosynthesis exothermic or endothermic?
Photosynthesis absorbs energy
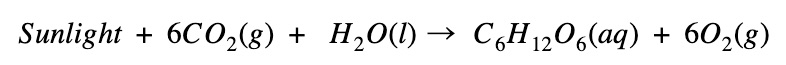
- In photosynthesis, energy is absorbed to create glucose (C6H12O6)
- The energy of the products (glucose and O₂) is higher than the reactants (CO₂ and H₂O)
- Therefore the reaction is endothermic, indicated by a negative change in enthalpy
 Watch an effective endothermic reaction experiment by Professor Bob Burk
Watch an effective endothermic reaction experiment by Professor Bob Burk


One thought on “Enthalpy”