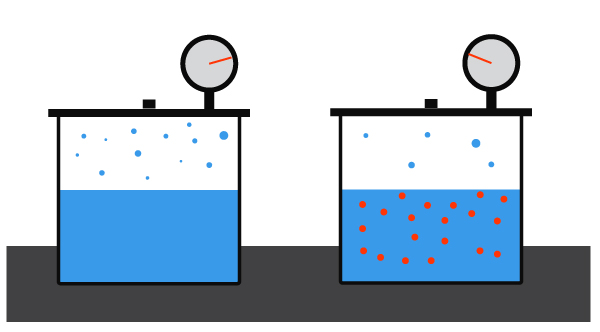
Adding a solute to a pure solvent lowers the solvent’s vapor pressure. For example, water normally boils at 100ºC (212ºF) but if you add a substance like salt to the solution, it decreases the vapor pressure of the solution.
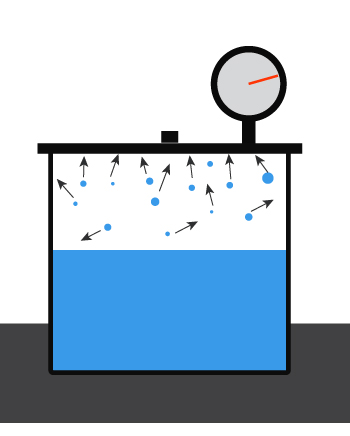
First, recall what the vapor pressure of a liquid is. For a liquid inside a closed container at a given temperature, some amount will evaporate and bounce around, hitting the walls of the container.
The pressure exerted by the liquid’s vapor
is the liquid’s vapor pressure.
Solute effect on evaporation rate
How quickly a liquid will evaporate is dependent on the amount of exposed surface area. In a cup in of pure water, the exposed surface is entirely made up of water molecules. However, if a solute like sugar were mixed with water, some of the molecules at the surface will be sugar. This effectively lowers the exposed surface area of the water, making it less able to evaporate and in turn lowering the vapor pressure.
- The water will only boil if its vapor pressure is equal to atmospheric pressure. Thus you would need to heat the water to a higher temperature.
- The vapor pressure lowering is a colligative property because it depends on the ratio of solute particles to solvent and not the identity (type) of solute.
- The more sugar you add to your cup of water the less surface area that is exposed and hence affects the vapor pressure of the liquid.


I’m a French student at Science University and It was quite difficult for me to understand colligative property before I discover your website. 🙂 Thank you for your clear explanations and your nice illustrations !
Meilin, I’m glad you found this website helpful. Illustration is a very powerful tool for studying. Thank you again for your feedback and please support us by helping to spread the word!