
The solubility of a substance is the amount of that substance that will dissolve in a given amount of solvent.
The difference between solute and solvent
- SOLUTE – the substance to be dissolved
- SOLVENT – the liquid for dissolving the substance
Fizzy drinks
Fizzy drinks are made by dissolving carbon dioxide gas in water.
The carbon dioxide forms a weak acid called carbonic acid (H2C03) in the solution.
Besides carbon dioxide, sugar is also dissolved in the water to give the drink a sweet taste.
Can you differentiate the solute from the solvent in the fizzy drink?
| Substance | Solute or solvent? |
| Carbon dioxide, CO₂ | Solute
|
| Water, H₂O | Solvent
|
| Sugar, C₆H₁₂O₆ | Solute
|
Classifying a substance as soluble
A substance is classified as soluble if more than 0.1 g can be dissolved in 100 mL of water.
Temperature
The solubility of a substance is affected by temperature. As temperature increases, the increased in kinetic energy causes the solvent molecules to move faster and collide with the solid particles. This in turn causes the solute molecules to break apart and move further apart from each other. Thus more solutes will dissolve in water at a higher temperature.
Generally, solids are more soluble when the temperature increases. Gases, on the other hand, are less soluble when temperature increases.
For example, more amount of salt will dissolve in warm water than cold water. However, fizzy drinks stays fizzy longer if kept in the refrigerator where the temperature is lower.
A solubility graph
Every chemical substance which dissolves in water has a fixed solubility at a specific temperature.
The diagram below shows the solubility of a certain compound in water.
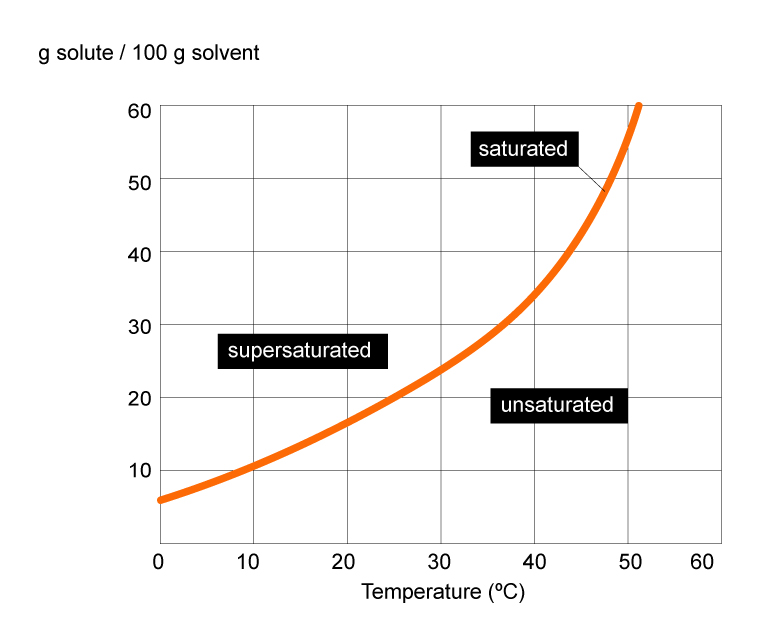
The solubility graphs shows that as the temperature increases, more solute particles will dissolve in the solvent.
The red line curve on the graph represents the saturation point for the solute. It shows the maximum amount of solute (in grams) that can be dissolved at a particular temperature.
Saturated solution
A saturated solution is a solution that contains the maximum amount of solute which can be dissolved in a given amount of solvent at a specific temperature.
In a saturated solution, adding more solutes to it will not dissolve.

