
Nonpolar molecules do not dissolve readily in water
- Nonpolar molecules do not become hydrated – they are repelled by water
- The molecules are insoluble or almost insoluble in water
Oils and fats are examples of hydrophobic substances. For example, if you mix oil with water, the mixture will separate. The oil molecules are more attracted to each other than to the water molecules.
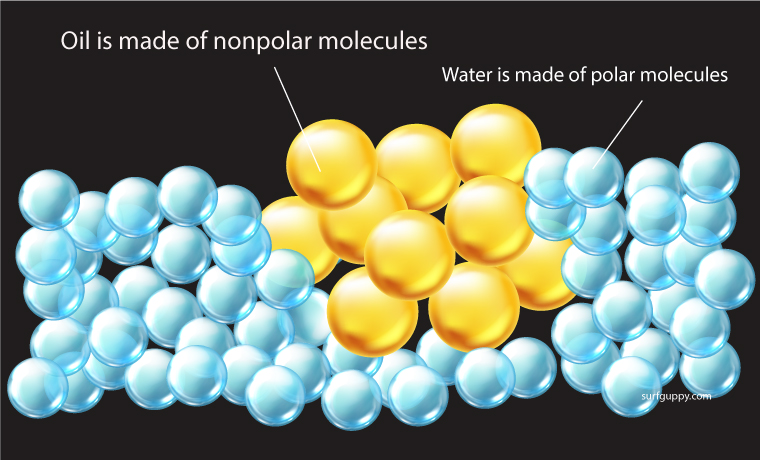
Nonpolar molecules are not attracted to water

