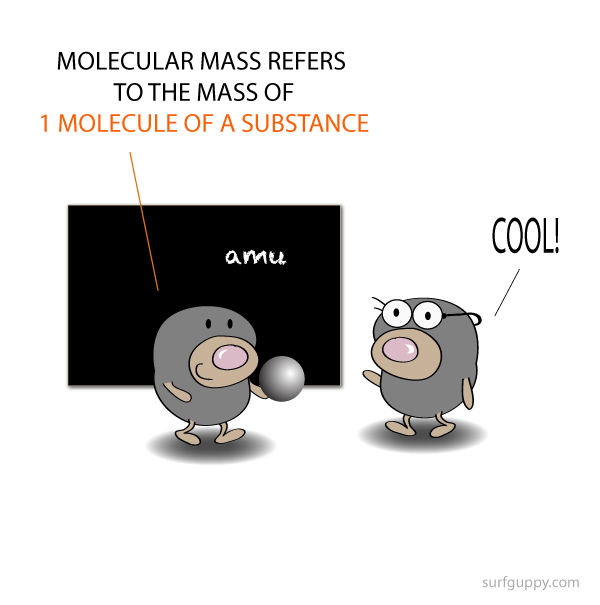
How to calculate molecular mass?
- Determine the formula of the compound
- Use the periodic table, determine the atomic mass of each element present in the formula
- Multiply the atomic mass of each element by the number of atoms
- Finally, add the atomic masses
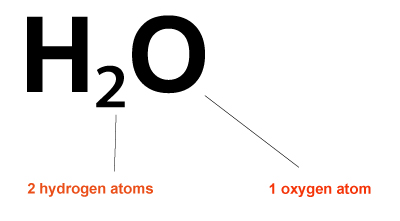
Example: How to find the molecular mass of water?
- First, determine the formula for water.
- The formula for water is H2O.
- Use the periodic table to look up the atomic mass for the elements in the formula.
- You must then count how many atoms of each type are present using the formula.
| ELEMENT | ATOMIC MASS | #ATOMS | TOTAL |
| hydrogen | 1.008 amu | 2 | 2(1.008) = 2.016 amu |
| oxygen | 16.00 amu | 1 | 1(16.00) = 16.00 amu |
| Total the column
= 18.02 amu (rounded) |
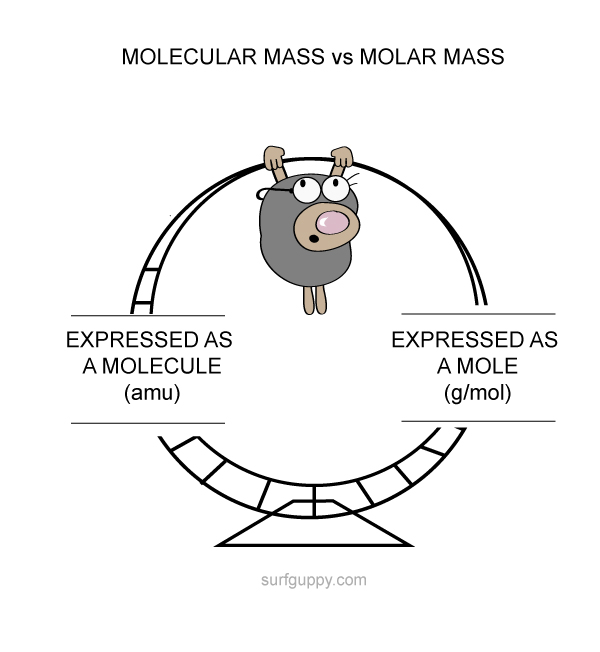
Molecular mass can also be expressed in terms of moles (g/mol)
| Substance | Formula | Molecular Mass | Molar Mass |
| Water | H2O | 18.02 amu | 18.02 g/mol |
| Carbon dioxide | CO2 | 44.01 amu | 44.01 g/mol |
| Oxygen | O2 | 32.00 amu | 32.00 g/mol |
Amu and g/mol are equivalent to each other.
The difference between molecular mass and molar mass


Beautiful presentation. Aren’t computers wonderful. When I taught chemistry, the best we had were calculators and overheads. You are so blessed. I’ll try to convey the way I approached the teaching of the mole. It would be easier to draw it than to type it, but I’ll try.
If you have an email I could send a written copy to you if you are interested in my approach.
dozen is 12; gross is 144; elom is 16. An object has a mass of 5 oz. What is the mass of an elom of them in pounds? 5 oz. x 1 lb. x 16 obj = 5 lbs.
1 obj 16 oz. 1 elom 1 elom
Since mole is 6.02 x 10 e 23 & there are 6.02 x 10 e 23 amu = 1 g, that’s why mass in amu is numerically equal to mass in grams { substitute amu for oz., grams for lb, and mole for elom in the above example. 16 was chosen for elom to numerically match 16 oz./lb So, 6.02 x 10 e23 was chosen to match 6.02 x 10 e 23 amu/g.}
The example of water: you speak about atomic weight, it must be atomic MASS!! (weight is a force: mass * gravity).
See you! Piet
Thanks Piet for submitting your comment! I’ve changed the term “atomic weight” to “atomic mass” to be absolutely clear.
Please see “Molecular Weight, Atomic Weight, Weight vs. Mass” in Chemwiki
The term “atomic weight” has historical roots, dating back to a time when the concept of atomic structure and isotopes was not well understood. In the early days of chemistry, chemists used the concept of atomic weights to describe the relative masses of elements in chemical reactions.
As our understanding of atomic structure advanced, it became clear that what we were actually measuring and calculating was the average mass of atoms of an element, which is more accurately described as atomic mass or relative atomic mass. The concept is fundamentally about mass, not weight in the sense of gravitational force.
So, while the term “atomic weight” has persisted in scientific language, the underlying concept is indeed mass. In modern chemistry, the distinction between mass and weight is important because mass is an intrinsic property of matter, while weight depends on the gravitational field in which an object is located. Atomic weight (or atomic mass) is a measure of mass, not weight, and it is expressed in atomic mass units (amu) or unified atomic mass units (u).
Ultimately, not to confuse young learners I think we will call it “atomic mass” instead 🙂