Dissociation is the breaking down of a compound into elements
The word dissociate and disassociate have similar meaning – to remove from being connected.
When a compound dissociates, the bonds are broken.
Ionic compound dissociation
All ionic compounds dissociate to some extent when they dissolve in water.
Ionic compounds are those compounds that consist of atoms called ions with opposite charges.
When an ionic compound dissociates in water, the ionic bond is broken.
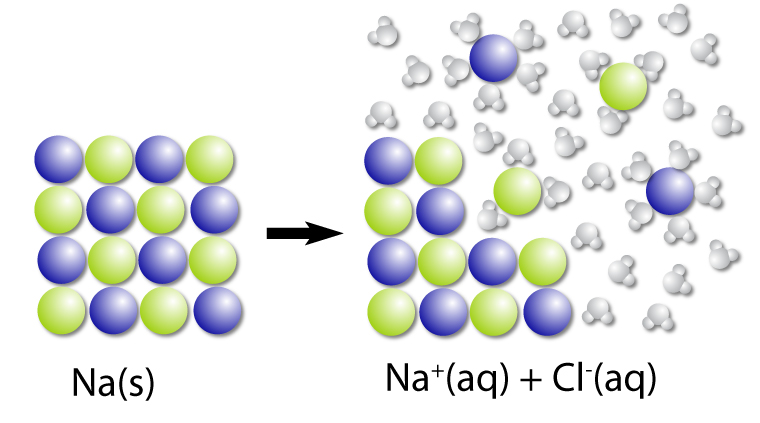
Dissociation of NaCl
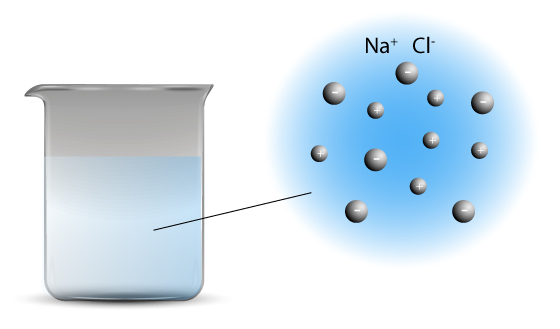 |
Solid sodium chloride dissolves in water to produce Na+ and Cl– ions.
The dissociation of NaCl can be written as:
NaCl(s) → Na+(aq) and Cl–(aq)
- (s) represents the solid state
- (aq) represents an aqueous solution
- An aqueous solution is a solution that has water as the solvent
Covalent compound dissociation
Generally, covalent compounds do not dissociate when dissolved in water. This is true for most cases. However there are some covalent compounds (such as hydrochloric acids) dissociates when dissolved in water.
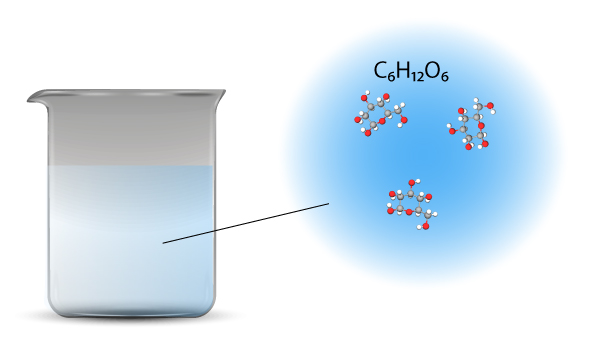 |
Glucose is a covalent compound. When dissolved in water, it does not dissociate. The molecules are separated in water – they move further apart but no bonds are broken. Each glucose molecule remains as a whole unit in water.
For more reading, please refer to sodium chloride dissociation in the process of solvation.

