Molar mass is the weight in grams of one mole of a chemical substance.
How to calculate the molar mass of a single element (eg.hydrogen)?

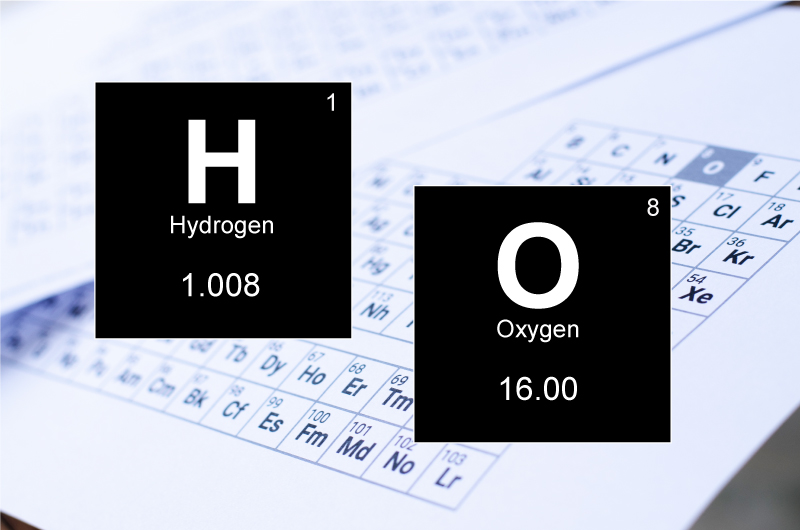
The molar mass for an element is the sum of atomic masses, expressed as g/mol.
Molar mass of hydrogen = 1.008 g/mol
How to calculate molar mass of a compound (eg. water)?
The molar mass for a compound can be calculated using the sum of the atomic masses of the constituent atoms, expressed as g/mol.
Molar mass of water
Formula: H2O
2(1.008) + 15.999 = 18.015 g/mol
How to calculate the molar mass of sucrose?
Formula: C12H22O11
We will use a table to show how to calculate the molar mass of sucrose:
| Elements | Symbol | Atomic Mass (amu) | Molar Mass (g/mol) | #number of atoms |
| Hydrogen | H | 1.008 | 1.008 | 22 |
| Carbon | C | 12.01 | 12.01 | 12 |
| Oxygen | O | 16.00 | 16.00 | 11 |
The molar mass of sucrose
= [22(1.008) + 12(12.01) + 11(16.00)] g/mol
= 342.30 g/mol

