The characteristics of a solution such as the boiling point, freezing point and vapor pressure are known as colligative properties. Colligative properties apply only to solutions and not gases or solids.
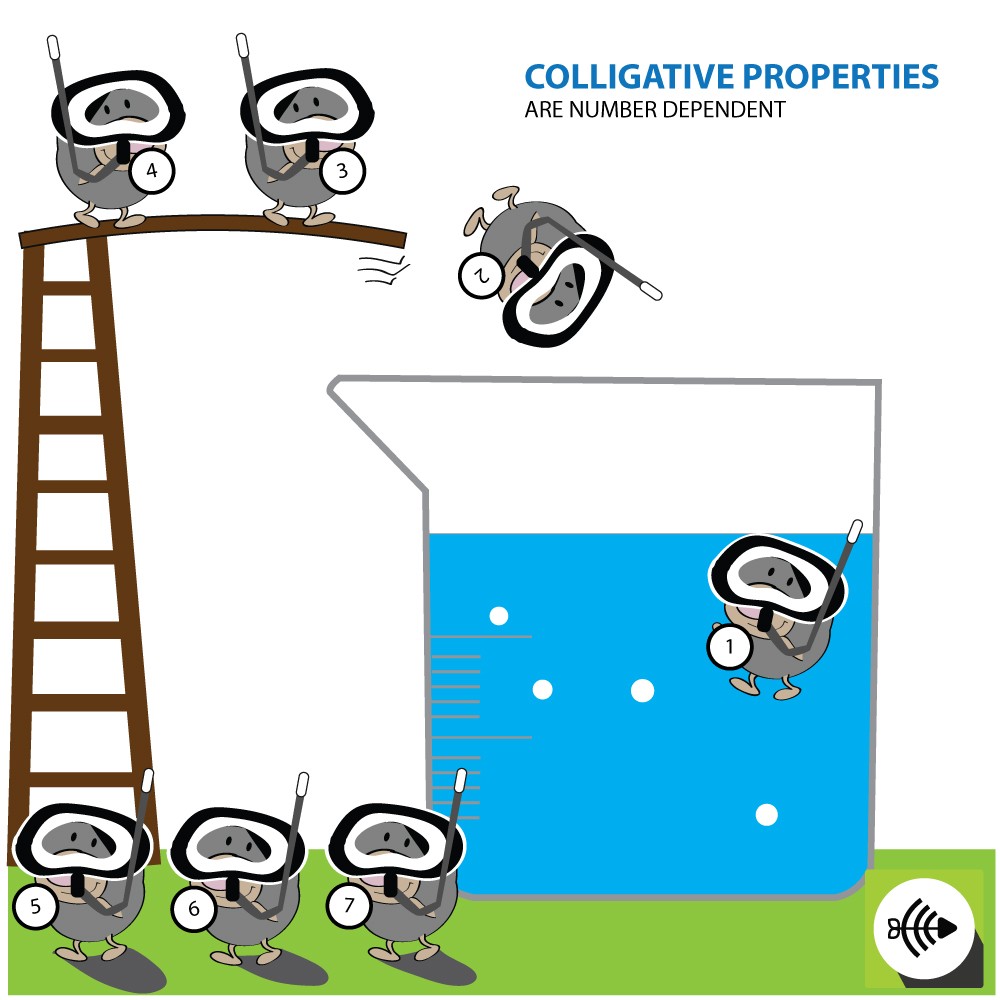
COLLIGATIVE PROPERTIES ARE DEPENDENT ON THE NUMBER OF ATOMS AND MOLECULES
The word colligative comes from the same Latin root that the word collection comes from.
Colligative properties are affected by the amount of dissolved particles in a solution and not the type of particles.
The change in temperature is calculated by:
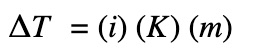 |
ΔT = change in temperature (ºC)
i = van’t Hoff factor
K represents Kf or Kb
Kf = molar freezing point depression constant (ºC kg/mol)
Kb = molar boiling point depression constant (ºC kg/mol)
m = molality of the solute in (mol/kg)
The van’t Hoff Factor
The van’t Hoff factor (i) is defined as the number of particles each unit of solute breaks apart into when it dissolves in a solvent.
| Substance | van’t Hoff factor (i) |
| Glucose (C6H12O6) | 1 |
| Salt (NaCl) | 2 |
| Magnesium chloride (MgCl2) | 3 |
For glucose (C6H12O6), the van’t Hoff factor is 1.
- The sugar molecule remains intact as a molecule in solution. It does not break apart, so the van’t Hoff factor is 1.
For salt (NaCl), the van’t Hoff factor is 2.
- A unit of NaCl breaks apart into 2 particles, Na+ and Cl– in solution. So the van’t Hoff factor for NaCl is 2.
Example #1
What is the freezing point of a 0.20 m concentration salt solution? Given the Kf for water is 1.86 oC/m.
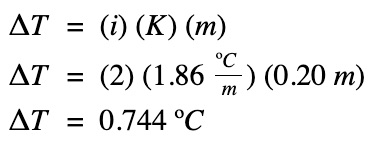
The freezing point of the salt solution:
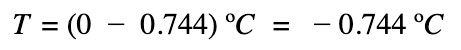
Example #2
What is the freezing point of the same solution if the concentration is halved?
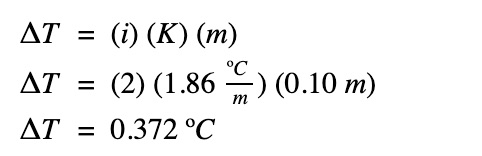
The freezing point of the salt solution:
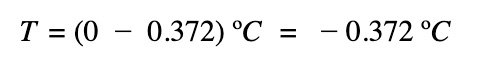
Remember, the freezing points of solutions are lower than that of the pure solvent and the higher the concentration of the solution, the more impact it has on the freezing point lowering.
RELATED TOPICS
| vapor pressure lowering |
| boiling point elevation |
| freezing point depression |

