
- When water flows over a dam, it loses some of its useful energy as heat
- Solid wood burns to become ash and releases gases.
- Solid ice melts to form liquid water.
- When converting the useful energy to do work, some of the energy is always lost as heat.
- The increase in unusable energy over time is referred to as entropy.
Entropy
First of all, entropy is the concept that everything in the universe tends towards disorder. For example, all living things age and die, they don’t get younger. Mechanical things tend to wear out and break down. Bricks are not naturally stacked up nicely or become part of a building unless someone has put a lot of effort into making it. So, disorder is more probable in the universe than order.
Entropy Change from Viziscience on Vimeo.
Thermodynamics
Thermodynamics is just a branch of physics that studies heat and other forms of energy (mechanical, electrical or chemical) and their relationships between each other.
1) The Law of Conservation
-
- The First Law of Thermodynamics states that matter/energy cannot be created or destroyed.
2) The Law of Increased Entropy
-
- The Second Law of Thermodynamics states that matter/energy gradually deteriorates over time.
While quantity remains the same (first law), the quality of matter/energy deteriorates. As energy is used for productivity – such as growing new cells or repairing tissues, useful energy is converted to unusable energy.
The Second Law of Thermodynamics states that once energy is changed from usable form to unusable form, it cannot be recovered.
Entropy is a measure of that unusable energy.
- When unusable energy increases, it means entropy also increases.
Energy transfer is studied in three types of system. It is important to understand what they are.
- Open
- Closed
- Isolated
Click here for more information on Thermodynamic Systems![]()
Entropy and disorder
There are quite a few variations for the definition of entropy. One idea that often leads to misconceptions is that “entropy is a measure of disorder.” One tends to think about patterns when the word “disorder” or “randomness” is used.
As described earlier, how do you measure disorder? Is it by how far molecules are apart from each other? Is it by the arrangement or pattern of molecules, particles, or objects? If you have a pack of cards randomly spread out a table next to a pack of cards neatly stacked, you may say one is more random than the other.
Below, group B & C cards are more randomly placed, but the properties of the cards have not changed. All three groups still have the same arrangement of molecules more or less and they have not undergone any heat or energy redistribution. So, a change in entropy has not taken place in any of the three groups of cards regardless of how they are arranged.
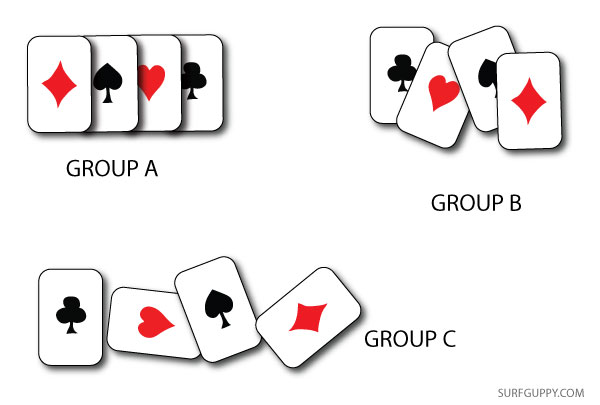
So, what is a good definition for entropy? A good approach is to think in terms of heat or energy redistribution, not randomness. If you decide to burn one set of cards, now you’re talking! The set of cards that you decide to burn would undergo an entropy change.
First, to burn the cards, you apply activation energy by means of lighting a match to it. The cards would then catch fire and burn spontaneously. The cards made of paper have potential chemical energy. Burning objects causes the potential energy in the materials to change. Some of the energy is transformed into thermal energy and dispersed into the atmosphere. Entropy is said to have increased. Overall, the burning of the set of cards adds to the entropy of the universe slightly. So, the more people burn cards, the more the entropy is increased in the universe!
When energy is dispersed, useful energy is turned into unusable energy. In burning a pack of cards, the bonds in the molecules of the cards are broken and new molecules (eg, carbon dioxide and water vapor) are made. The heat produced from the process escapes into the atmosphere. Once the burning has stopped, the temperature difference between the cards and surrounding air evens out and thermal energy movement ceases. Heat can only move from a place of higher temperature to lower temperature. If everything is at the same temperature, then heat energy cannot transfer. At this point you can say work is done – completed! Equilibrium has been reached and you won’t need to call the fireman out to kill the fire.

You can see now that as usable energy decreases, unusable energy increases. The amount of unusable energy generated as a result of the process is related to entropy. So entropy increases when the amount of unusable energy increases. For the burned cards, no matter how long you wait, the chances of them becoming “unburnt” are zero. Energy cannot flow back into the ashes to make the cards whole again by a natural process.
The Second Law of Thermodynamics states that entropy in the universe always increases. This is true if the universe is an isolated system, where you would be unable to put in or remove energy. It starts off with a certain amount of energy, and due to decay, all the usable energy will eventually be converted into unusable form. So even if you don’t burn the cards, over time, after many years, perhaps thousands or millions of years, the cards will eventually disintegrate. So, entropy increases over time. In that sense, everything tends towards disorder. Not disorder in the sense of the arrangement or patterns of things, but in the breaking down and degeneration of matter from usable to unusable energy. You can understand why scientists say time cannot go backwards; it only proceeds forward!
So far, the discussion above says that disorder doesn’t mean the pattern or arrangement of particles. But sometimes the arrangement of particles can tell you about entropy. Gas particles have random motion have high entropy. Solids have little random motion have low entropy.
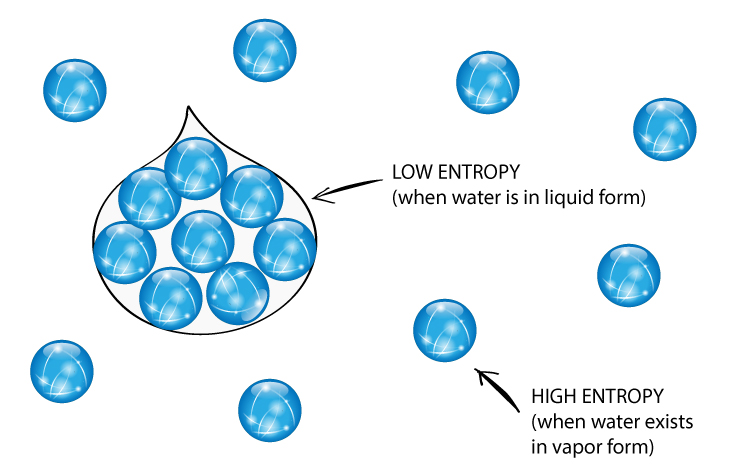
Measuring entropy
One useful way of measuring entropy is by the following equation:
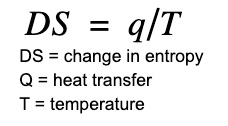
Using this equation it is possible to measure entropy changes using a calorimeter. The unit of entropy is J/K.
Predicting changes in entropy
How do you predict if a chemical or physical change is going towards orderliness or randomness? There are three principles you can apply.
| Ways to increase entropy | Description |
| Changing states
Entropy becomes greater changing from solid to liquid, and liquid to gas (s) —> (l) or (l) —> (g) |
The molecules in solids are packed together, making the solid rigid. The molecules in liquids experience less attraction to one another and as a result, liquids can fit to the shape of their container. The molecules in gases experience little to no attraction to each other and as a result, gases can fit to the shape of their container and expand to match the volume of their container as well. |
| Changing from a solute to an aqueous solution
Entropy increases when you dissolve a solid in a liquid, or combining a liquid into a solution with other solvent. (s) —> (l) or (l) —> (g) |
It increases in entropy because the solute particle can move more freely and randomly in a solution. |
| Increasing the number of particles
The more particles (especially in a gaseous state) the greater the number of microstates. (g) —> 2(g) or 2(l) —> 3(l) |
Example, in a chemical reaction, increasing from 2 moles of particles (on the reactant side) to 3 moles of particles (on the product side) increases the entropy.
Example: 2SO₃(g) → 2SO₂(g) + O₂(g) |
Entropy of a system
ΔSsystem > 0 Increase in entropy
ΔSsystem < 0 decrease in entropy
| (l) → (s)
liquid to solid |
ΔS < 0 |
| (s) → (l)
solid to liquid |
ΔS > 0 |
| (s) → (g)
solid to gas |
ΔS > 0 |
| (g) → (l)
gas to liquid |
ΔS< 0 |


How the entropy of universe will increase?it should remain constant right?As system+surrounding=universe. So one(system or surrounding)is losing entropy and other is gaining (system or surrounding) entropy. Therefore initial entropy of universe= final entropy of inverse
Why is it necessary to have unusable energy?
Entropy is often described as the measure of the dispersion or spread of energy. Systems naturally evolve in a direction that increases entropy. As energy disperses, a portion of it becomes less available for doing work—essentially, it becomes “unusable.” Therefore, during energy transformations, entropy usually rises, signifying a portion of the energy dispersing and becoming “unusable.”
This inevitable rise in entropy powers many natural processes. For example, the melting of ice into water isn’t merely a function of temperature. The process also involves an entropy increase, as water molecules in a liquid state exhibit greater “disorder” than those in a solid state. This “disorder” means that molecules move more freely and randomly in various directions. Many chemical reactions, similarly, favor a direction that results in an increase in entropy.