Open, Closed & Isolated Systems
A system refers to any parts of the universe being studied.
If you are conducting an experiment in a beaker, then the system you are studying is in the beaker.
The system is subject to surrounding factors such as air temperature and pressure.
Thermodynamics involve the study of heat energy exchange between a system and its surroundings.
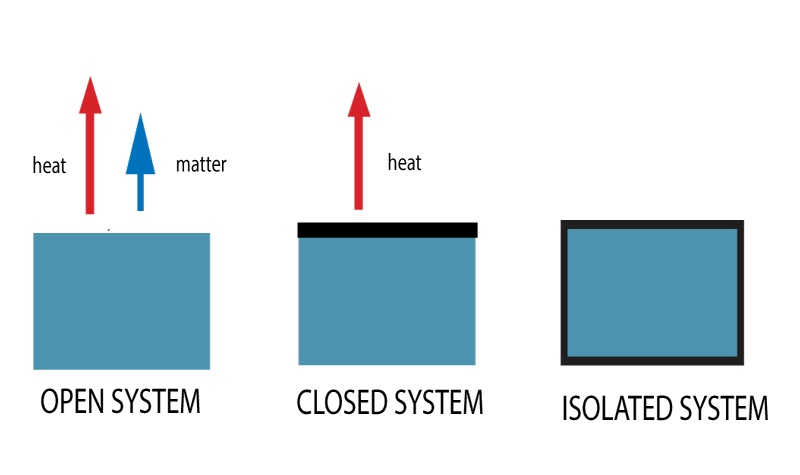
There are three types of thermodynamics systems. Based on the possible heat and matter transfer, they are classified as open, closed or isolated systems.
Types of Thermodynamic Systems
Open Systems
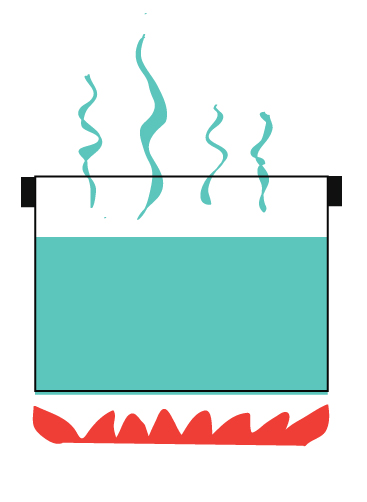
You may have heard of open systems and closed systems. An open system is one that freely allows both energy and matter to be transferred in an out of a system.
For example, boiling water without a lid.
Heat escaping into the air.
Steam (which is matter) escaping into the air.
Closed Systems
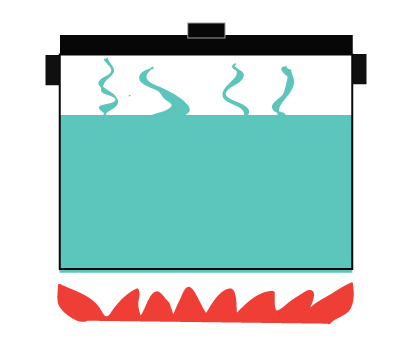
A closed system, on the other hand, does not allow the exchange of matter but allows energy to be transferred.
It allows heat to be transferred from the stove to the water
Heat is also transferred to the surroundings
Steam is not allowed to escape
Example of a closed system – a pressure cooker.
Nb: If a system is 100% closed, it is in danger of exploding. That's why a pressure cooker should be designed with safety mechanisms to prevent the system from over-pressurzing by allowing steam to escape when needed.
Isolated Systems
This system is completely sealed.
Neither matter nor heat can transfer to or from the surroundings.
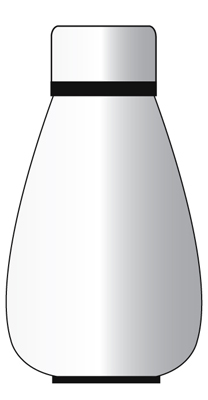 Example – A thermo flask.
Example – A thermo flask.
The purpose of a thermo flask is to keep your food warm.
A thermo flask can be considered an isolated system but only for a short period of time.
It prevents both heat and matter from being transferred to the surrounding.
Ultimately, the heat in the thermo flask will escape to the surroundings and the content inside the flask will be cooled down.


hello good people I am trying to find an example of isolated system in nature can anyone help
Hi Onalenna, thanks for your question. It’s very hard to find examples of isolated system in nature because isolated systems don’t exist in nature. However, the universe is considered an isolated system but that is based on the assumption that there is no other universe adjacent to this one that we’re living in and our universe is not exchanging energy and matter with any “surroundings”. Here’s one of an isolated system in physics that is quite abstract in concept – http://www.physicsclassroom.com/class/momentum/Lesson-2/Isolated-Systems.
In nature there is no real isolated system exist but universe is good example for isolated system
There is no isolated systems in nature.
Correct – there is no isolated systems in nature. But the universe could be considered an isolated system as the universe has not been observed to exchange energy or matter with something outside of it.
Adiabatic has no heat or matter entering or leaving the system. While isolated system has neither matter nor energy entering or leaving the system
Thermos bottle is the right example of isolated system
gas tank is a good example
these do not exist in nature sadly
‘Universe’ is the best example for an isolated system
Please i need a labeled diagram for isolated system
Boundaries: The solid line surrounding the system.
Contents: Represented by “Energy Content” and “Matter” within the system.
Draw a rectangle for the system.
Label the rectangle “Isolated System“.
Inside the rectangle, make note of the “Energy Content” and the type of “Matter” (gas, liquid, solid) present.
The boundaries are the sides of the rectangle, emphasizing that nothing is entering or leaving the system.
This is a very basic representation. Depending on the specific context or details you’re discussing, you might need to add more components or details to the sketch.
Remember – The concept of an isolated system is largely theoretical, and it serves as an idealization in thermodynamics to simplify discussions and calculations. In the real world, it’s extremely challenging, if not impossible, to create a perfectly isolated system due to various forms of energy like radiation, gravitational forces, and even minute thermal leakages.
How different is adiabatic system and isolated system ?
A comparisons of the 4 systems.
Isolated system – no transfer of energy or matter at all
Adiabatic system – only transfer work
Follow this link to see an example – http://www.dummies.com/education/science/physics/keeping-a-system-at-constant-heat-the-adiabatic-process/
Hey, any chance I can get a publish date on this article, so I can reference it for my assignment?
I need one natural example of closed system, please someone help mi with an example of it?
Is photosynthesis a closed system or open system?
Hi people. Please can an opened system be isolated
See the definitions:
An open system can exchange matter and energy.
A closed system can exchange energy (e.g. heat) but not matter.
An isolated system cannot exchange energy or matter.
So, if your system is open, it lets both heat and matter out. It can be turned into an isolated system by closing and insulating it so neither heat or matter can escape. However, there are no truly isolated systems in the world. Some matter or heat will always escape from a container. In the case of a science experiment in school, you can assume a system such as the coffee cup calorimeter as an isolated system for the purpose of doing your calculations, but in reality it is not an isolated system.
An open system can exchange matter and energy. A closed system can exchange energy