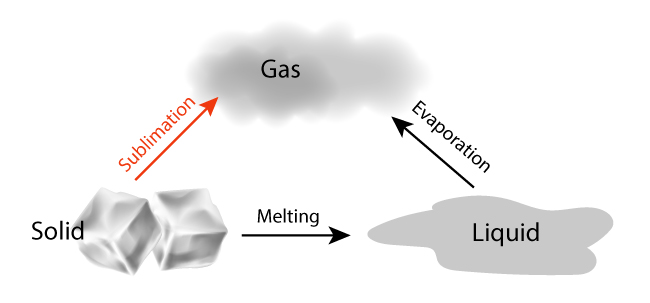
Sublimation is the process of turning from solid to vapor without going through the intermediate liquid phase.
Sublimation of Ice
We are all familiar with ice melting before it evaporates. However, in sublimation, the liquid phase is omitted. In order for a solid to sublime, it must exhibit a higher than usual vapor pressure and weak intermolecular attractions.
Have you ever noticed ice cubes left in the freezer shrink over time?
The freezer has the right condition for ice cubes to sublime – i.e. to turn into water vapor without first melting.
The factors affecting sublimation are:
- Temperature
- Humidity
Low temperatures lead to air with low humidity (dry air), which has a lower vapor pressure that more closely matches that of the ice. So, molecules in the ice cubes are able to overcome the intermolecular forces which hold them together and are able to move far apart from each other and if there is sufficient energy the molecules on the surface of the ice escape into the atmosphere.
Dry Ice
A solid carbon dioxide compound can sublime at 1 atm atmospheric at 78.5°C.
Picture: Richard Wheeler (Zephyris) at en.wikipedia, CC BY-SA 3.0


I have been away from chemistry for more tears than I care to admit. I wish that I had been able to utilize online refresher courses like this one, but I really enjoy getting back to my beloved chem. Thanks