Condensation polymers are any kind of polymers formed through a condensation reaction where molecules join together–losing small molecules as by-products such as water or methanol.
en.wikipedia.org/wiki/Condensation_polymerExample – converting ethylene terephthalate to polyethylene
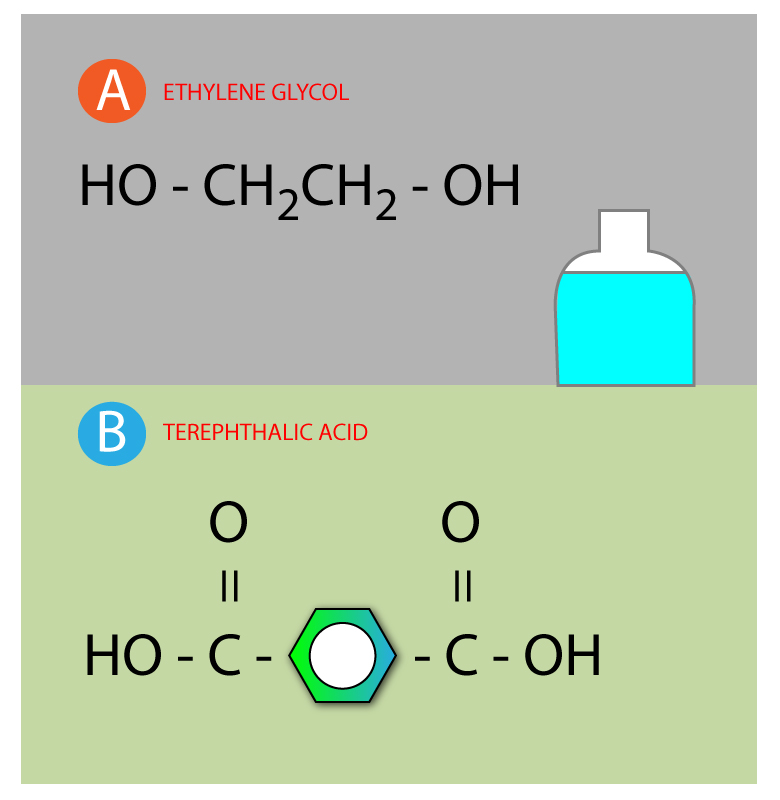
Step 1: Draw two different monomer molecular structure
MOLECULE A AND MOLECULE B
 This pretty symbol you see is called an ester linkage formed by combining acid and an alcohol. You don’t have to worry about it, just draw it in 🙂
This pretty symbol you see is called an ester linkage formed by combining acid and an alcohol. You don’t have to worry about it, just draw it in 🙂
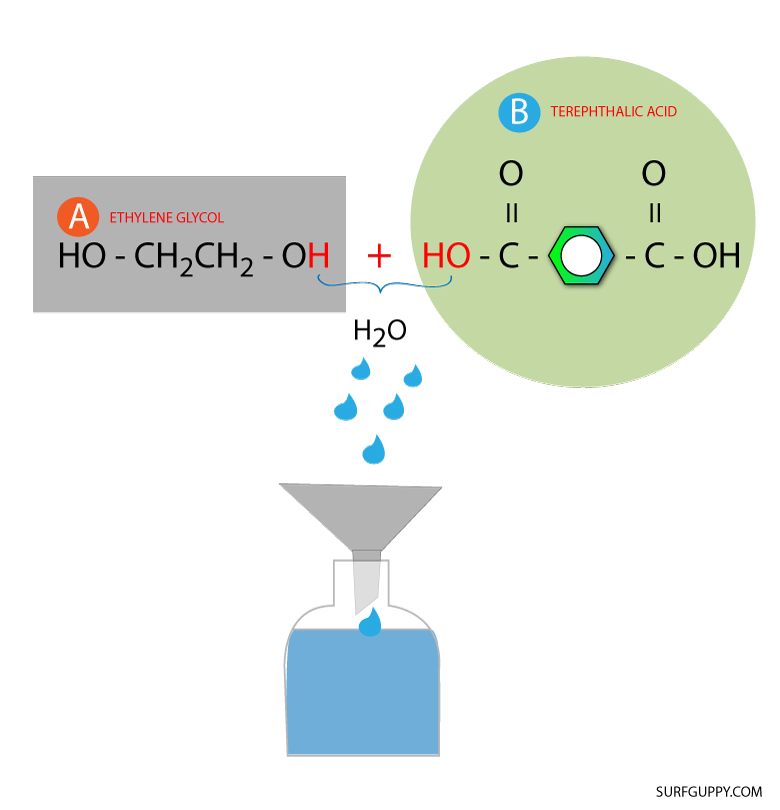
Step 2: Combine the monomers and remove the water molecule
The combination produces a water molecule
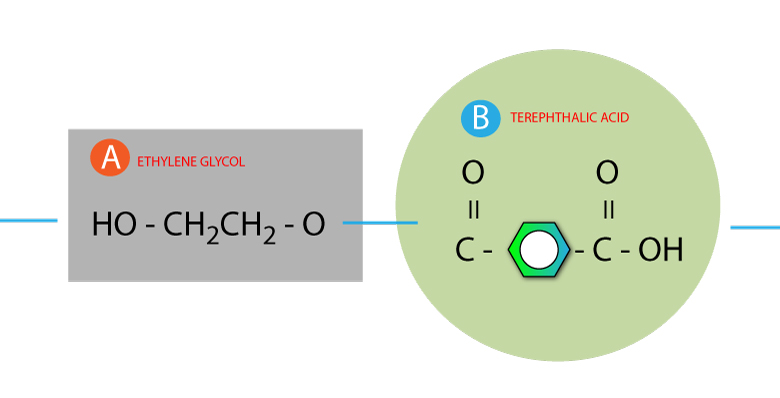
Step 3: String the molecules together without the water
Make a continuous string of molecules, below shows an example of 2 molecules linked together.
Step 4: Make polymer with Nth term
You can join lots of molecules together and keep repeating the structure.
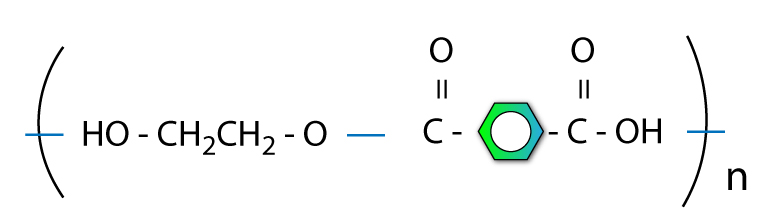


This is good.
I was looking for the simplest molecules [i.e. with least number of atoms]
to teach condensation polymerization to sophomores who are not Science Majors.