
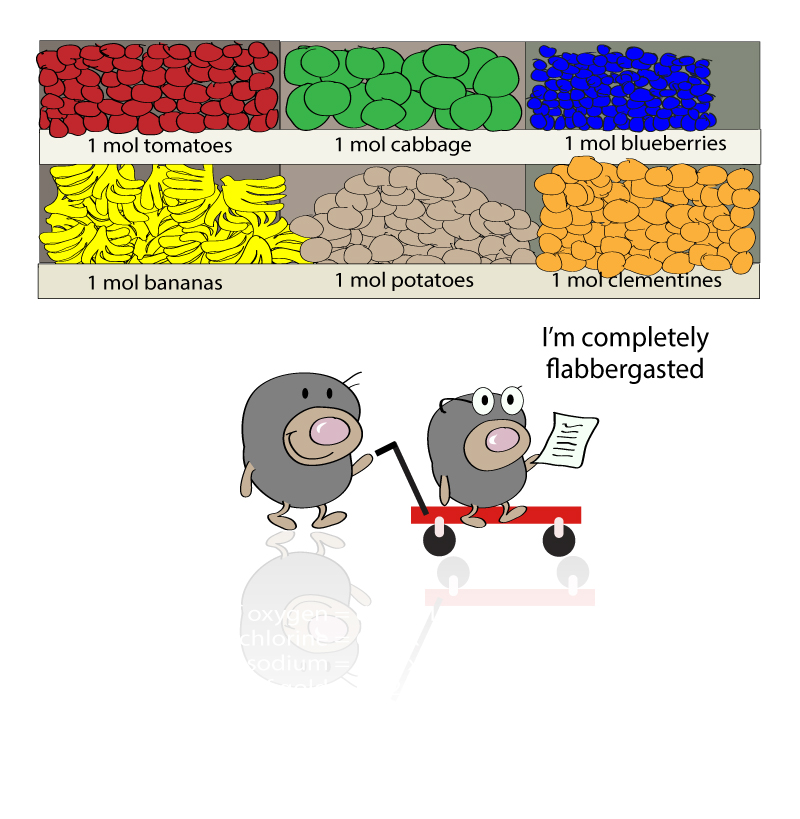
The mole is used in measurements in a way similar to the use of “dozens”.
- The reason why we use moles in chemistry is because of the vast number of molecules and atoms involved in the calculations
- Just like it’s easier to manage the thought of 2 dozen eggs instead of 24, scientists prefer to think of a reaction requiring 2 moles of carbon rather than 1,204,428,200,000,000,000,000,000 particles
- The number is huge and unimaginable, a bit like the number of stars in the galaxy
- Using the mole unit helps simplify calculations
Note: the symbol for mole is “mol”
The mole concept

The mole is equal to 602 thousand billion billion!

1 mole = 602,000,000,000,000,000,000,000
AMADEO AVOGADRO

Amadeo Avogadro was an Italian physicist best known for his hypothesis that equal volumes of different gases contain the same number of molecules at the same temperature and pressure. It was rejected by other scientists of his time and consequently, the mole wasn’t introduced until long after his death.
Remember, the mole, also known as Avogadro’s constant, is a representation of the number of atoms found in 12 grams of carbon . It’s important to memorize this number (6.022 x 10^23 ) so you can easily recall it for your calculations.
Mole & Mass
It is important to know that 1 mole represents the same number but not the same mass.
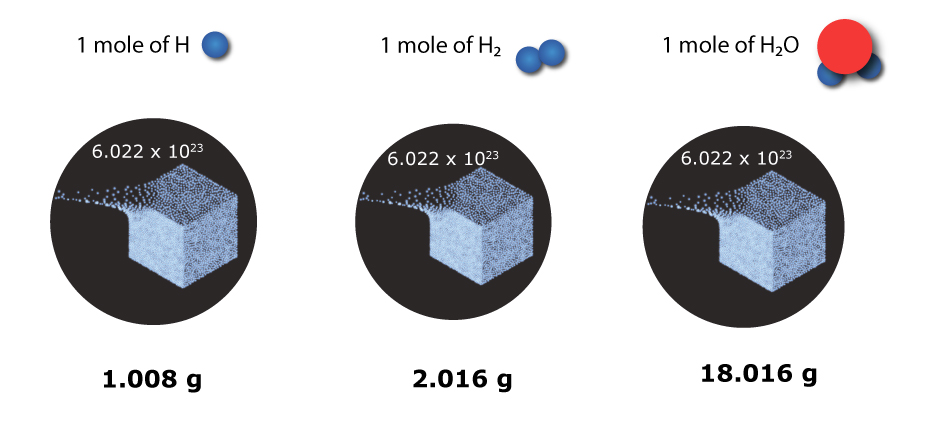
| Species | Total number of items
in 1 mole |
Total mass in
1 mole |
H |
6.022 x 1023 H atoms |
1.008 g |
H2 |
6.022 x 1023 H2 molecules |
2.016 g |
H2O |
6.022 x 1023 H2O molecules |
18.016 g |

