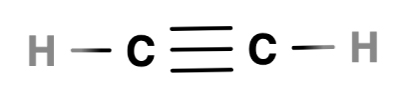
Carbon-carbon bonds
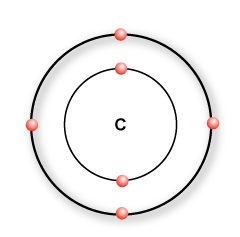
A carbon atom has 4 valence electrons
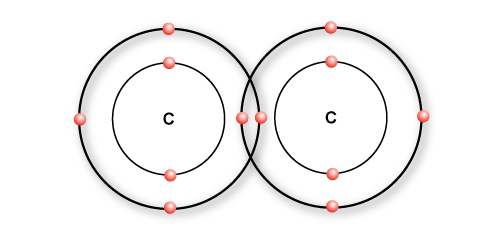
A single bond
Most bonds that carbon form are single bonds. Single bond simply means sharing one pair of bonding electrons between two atoms and is represented by a single line.
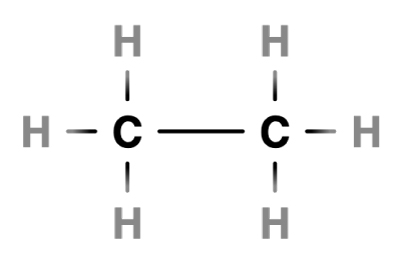
Two carbon atoms forming a single bond
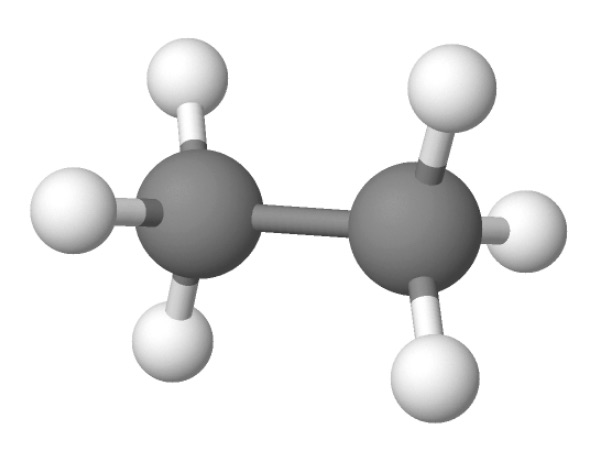
In ethane, two carbons atoms share a single bond
A double bond
A carbon atom can also form multiple bonds with other carbon atoms.
In a double bond, two pairs of electrons are shared.
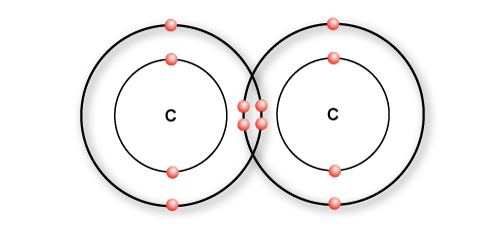
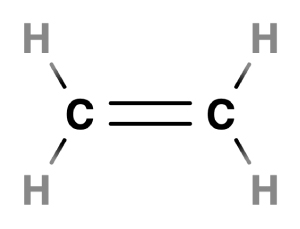
In ethylene, a double bond is formed between two carbon atoms
A triple bond
In a triple bond, three pairs of electrons are shared
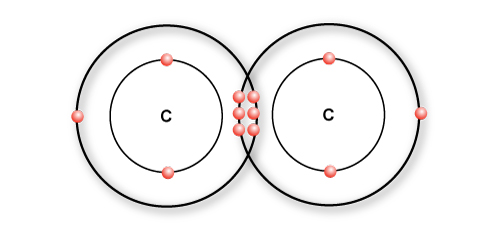
In ethyne, a triple bond is formed between two carbon atoms