Methane gas – CH₄
When a chemical reaction takes place, heat is either given off or absorbed.
- If heat is given off, it is called an exothermic reaction.
- If heat is absorbed, it is called endothermic reaction.
In this chapter we will learn how to calculate the enthalpy change (heat change) when methane (a gas) is formed using carbon graphite and hydrogen.
Hess’s law
Hess’s law is used to determine the enthalpy change. At this stage we don’t know if the process is exothermic or endothermic but we can find out based on the value of enthalpy change.
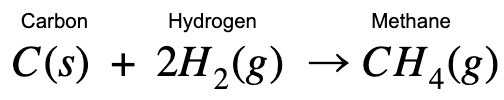
Given the enthalpy change of the following combustion processes:
| Combustion reactions | ΔH (kJ/mol-rxn) |
| carbon combustion | -393.5 |
| hydrogen combustion | -285.8 |
| methane combustion | -890.3 |
You may be wondering why you’re given the values of enthalpy change for the three combustion processes. This is because you can derive enthalpy change for methane formation by adding those three combustion together using Hess Law.
Watch a video – Enthalpy change for methane formation
Hess’s law – does not depend on path taken
Life would be so easy if we could directly measure the formation of methane from carbon and hydrogen. However, this can’t be done because the process is too slow to produce any meaningful results. This is why Hess’s law comes in handy. If you can use a sequence of multiple reactions to go from A to B, you can borrow the calculations to find out the enthalpy or heat change. As we explained earlier— it does not matter what route or how many steps, the overall enthalpy change is the same!

We can do just this for the formation of methane! Instead We can conduct three different combustion processes which combine to form the overall reaction we would have liked to observe directly, that is, the formation of methane from carbon and hydrogen.
- combustion of carbon
- combustion of hydrogen
- combustion of methane
By recording the enthalpy change for these three processes, you can then solve for the enthalpy change of methane by rearranging the equations. Remember, according to Hess’s law, it doesn’t matter what steps or how many steps are taken, as long as you are going from A to B under the same pressure.
In Hess’s Law problems, we need to rearrange the chemical reactions so that they combine to form the reaction needed. This can be achieved using the following fundamental rules:
- A reaction or process can be reversed.
- If the reaction is reversed, the sign for the enthalpy ∆H (the values) must also be flipped.
- The reaction can be multiplied by a constant.
- The enthalpy ∆H must also be multiplied by the same constant as the reaction
Methane formation – see a diagrammatic view of the calculation
How to caluclate the enthalpy change for methane formation using Hess’s Law

