The Haber process to manufacture ammonia using nitrogen and hydrogen is given by the equation below:
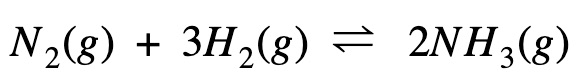
Calculate Gibb’s free energy change (ΔG) at 298 K temperature.
Given the entropy change (ΔS): -198 J K⁻¹ mol⁻¹
Enthalpy change (ΔH): -92 kJ
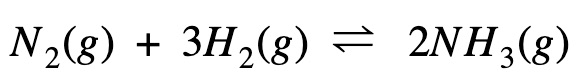
Calculate Gibb’s free energy change (ΔG) at 298 K temperature.
Given the entropy change (ΔS): -198 J K⁻¹ mol⁻¹
Enthalpy change (ΔH): -92 kJ
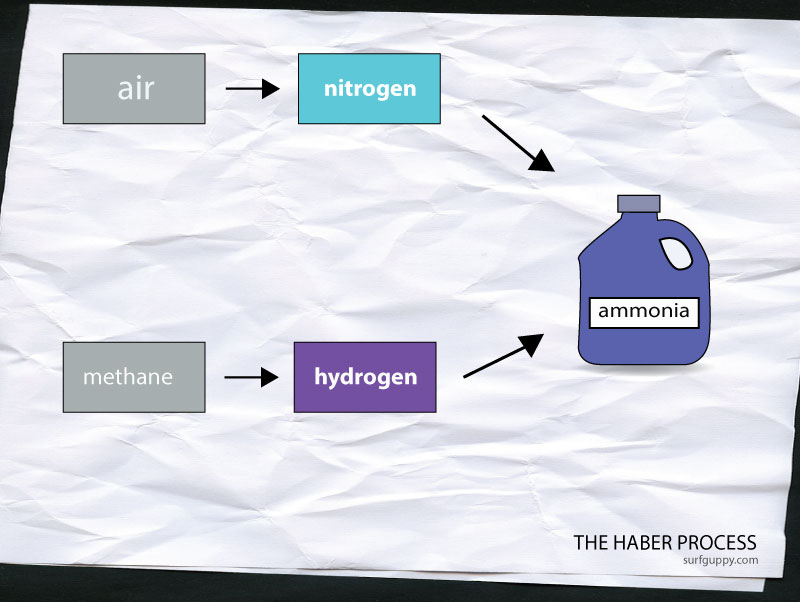
The Haber Process is a process of manufacturing ammonia in a factory. The raw materials are nitrogen and hydrogen. Nitrogen is obtained by burning hydrogen in air. Hydrogen is obtained by reacting methane with steam. Haber Process combines nitrogen and hydrogen to create ammonia. Ammonia is used widely in household cleaners and other applications such as fertilizers, explosives and dyes.
Steps for calculating Gibb’s Free Energy
The reaction
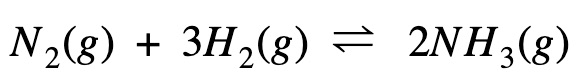
Gibb’s free energy

Convert (ΔS) to the right unit for calculations
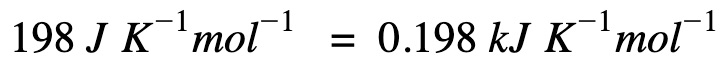
Apply Gibb’s free energy equation.
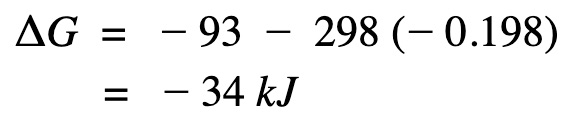
Gibb’s free energy is a negative value and therefore the reaction is spontaneous at that temperature.

