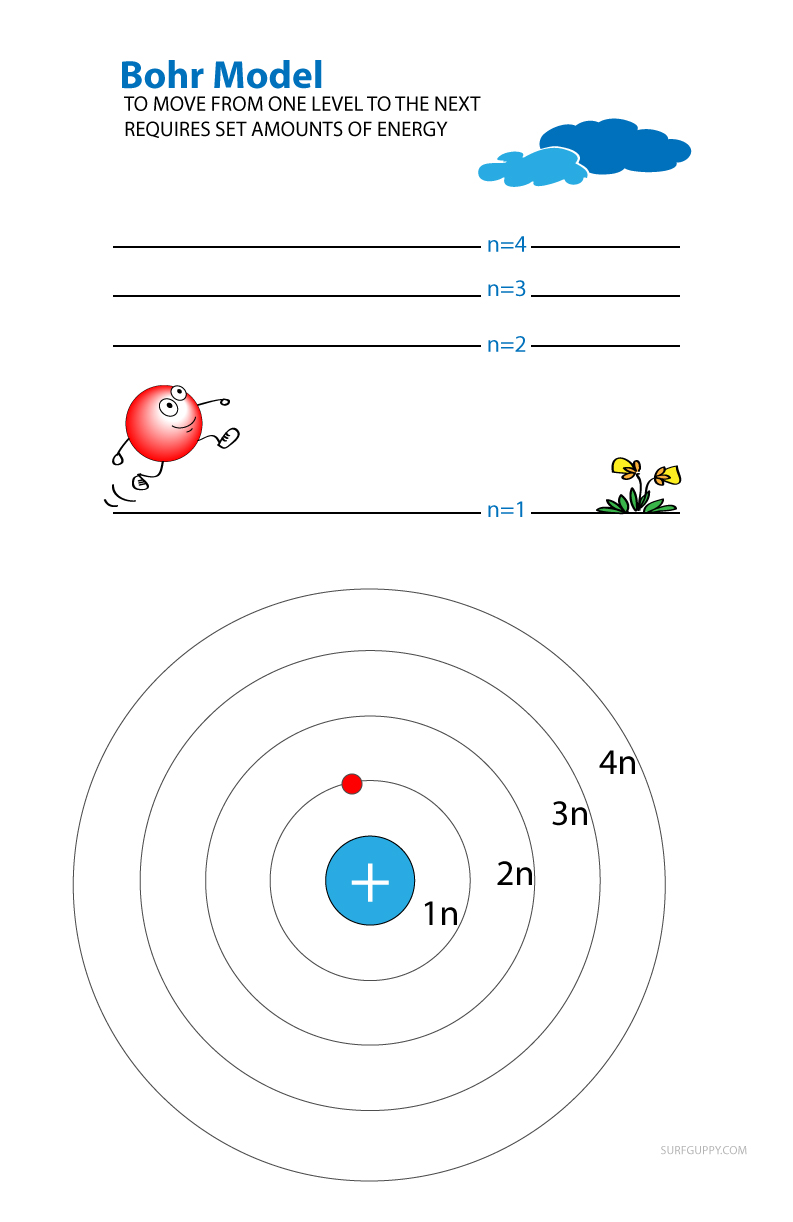
In 1913 Niels Bohr proposed a model to describe how energy levels are organized around an atom. While his model is now known to be incorrect, it is useful for understanding energy levels as Bohr’s model closely resembles planets orbiting the sun (a visual that we are familiar with).
According to the theory, electrons orbit the nucleus in rings (what we refer to as shells or energy levels).
- The innermost ring electrons (lowest energy) are bound the most tightly as they are very close to the nucleus and don’t have any electrons shielding them.
- Rings further from the nucleus are less tightly bound because they are both further away from the nucleus and also have other electrons in between them, shielding them from the pull of the positive nucleus.
- The further away from the nucleus, the higher the energy level.
Energy levels
If an electron were to absorb energy, would it go down in energy level, stay the same, or go up?
The answer is the electron would move up in energy level when absorbing energy. To move an electron from a lower shell to a higher one, energy is required. This is because the electron is attracted to the nucleus of the atom, and it needs energy to move further away from it.
When an electron absorbs enough light energy, it travels up an energy level. However, it may quickly become unstable and starts to lose energy. When this happens, the electron moves back down an energy level. This is called decay and the electron emits a photon of light.
In this theory, energy levels are given as n = 1, n = 2, n = 3 and n = 4.
- If an electron moves from n=1 to n=3, the amount of energy absorbed is equal to the difference in energy between level 3 and level 1.
- If it goes from level 4 to 3, then it loses the amount of energy equivalent to difference between level 4 and level 3.
Don’t confuse energy levels with orbitals. Energy level describes the shell or ring and covers an entire row on the periodic table. Orbital describes space where electron pairs are most likely to be found. One energy level can cover multiple orbitals.

