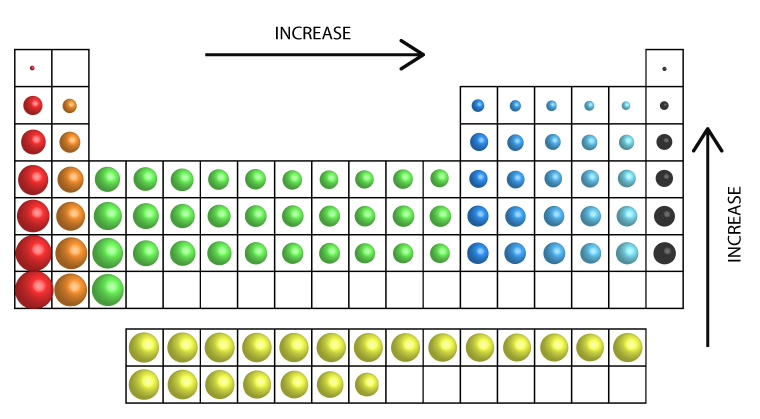
Ionization Energy Trend
Ionization energy is defined as the amount of energy needed to eject a valence electron from an atom in the gas phase. It is measured in kJ/mol.
A valence electron is simply an electron located in the outermost energy level of an atom.
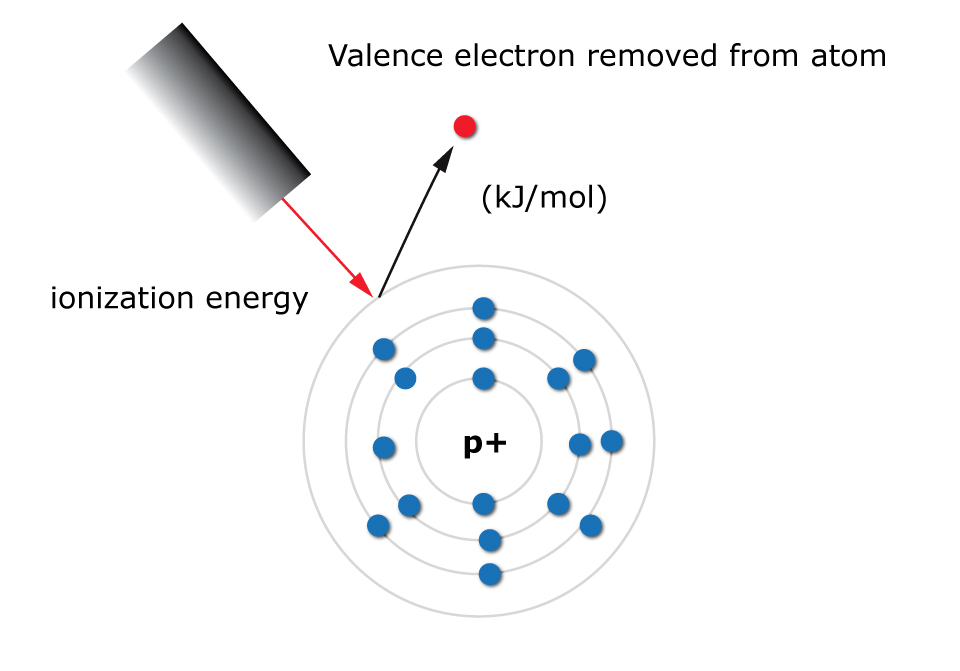
What is the ionization energy trend in the periodic table?
From the previous discussion, we know that going across a period (row) in the periodic table, the atomic radius decreases (i.e. atoms get smaller).
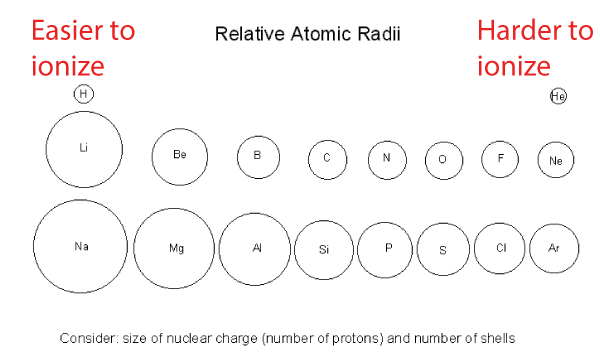
- As we move horizontally across the periodic table, one proton and one electron are added. The increasing number of protons pulls the electrons closer to the nucleus.
- This means more energy is required to remove the valence electrons that are now more tightly held
- Therefore, the ionization energy increases when atomic radius decreases
Ionization energy increases from left to right across the periodic table as atoms get smaller.
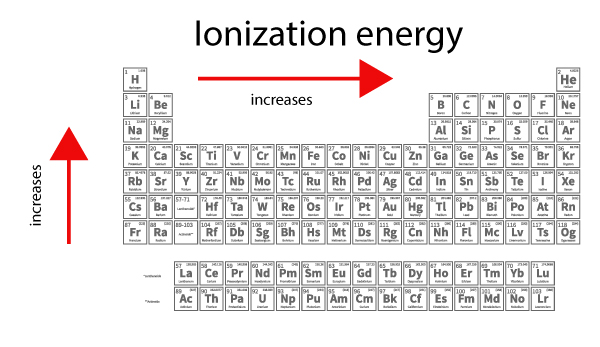
Can you explain the ionization trend within a group?
- The atomic radius gets larger going down a group because there are more layers of core electrons.
- These core electrons reduce the pull from the nucleus, so the outer electrons don’t feel the full force of the nucleus.
- As a result, the valence electrons are less tightly held and more easily removed, which is why the ionization energy decreases.


