First, you need to be clear what the system is and what the surrounding is. So you will need to define the system’s boundary.
We have an example here, HCl and NaOH reacting in a beaker. We want to measure the ΔH of reaction.
So, we can draw an imaginary boundary – shown by the dotted line. This will represent the chemical reaction taking place inside the beaker.
You will only be interested in the heat exchange between the chemical reaction and the surrounding.
So the chemical reagents are considered part of the system.
The rest you can consider the surrounding.
It’s very hard to measure the change in temperature of the reaction directly, so we measure the change in temperature of the surroundings.
So using the equation q = (m)(c)(T₂ – T₁), we can work out the heat change
Because the heat change is measured as part of the surrounding, we must reverse the sign to obtain the heat change of the reaction:
qᵣₓₙ = -qₛᵤᵣᵣ
Are beakers and thermometers examples of a system?
Are the reagents in an experiment also considered part of the system?
First, you need to be clear what the system is and what the surrounding is. So you will need to define the system’s boundary.
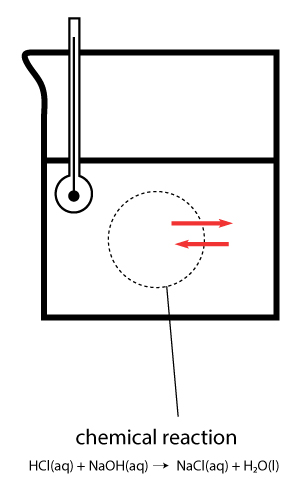
We have an example here, HCl and NaOH reacting in a beaker. We want to measure the ΔH of reaction.
So, we can draw an imaginary boundary – shown by the dotted line. This will represent the chemical reaction taking place inside the beaker.
You will only be interested in the heat exchange between the chemical reaction and the surrounding.
So the chemical reagents are considered part of the system.
The rest you can consider the surrounding.
It’s very hard to measure the change in temperature of the reaction directly, so we measure the change in temperature of the surroundings.
So using the equation q = (m)(c)(T₂ – T₁), we can work out the heat change
Because the heat change is measured as part of the surrounding, we must reverse the sign to obtain the heat change of the reaction:
qᵣₓₙ = -qₛᵤᵣᵣ