ELECTRONEGATIVITY
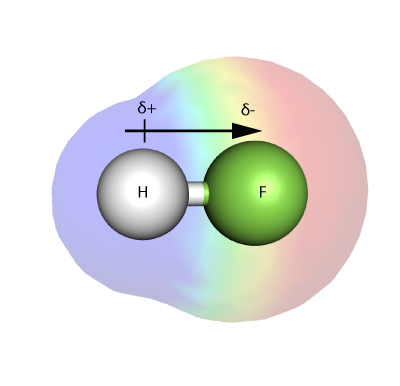
Electronegativity is a measure of how strongly an atom – in a molecule – is able to attract electrons in a bond to itself.
Factors affecting electronegativity:
1) The atomic number
The atomic number is the number of protons in the nucleus of an atom.
It determines the chemical properties of an element and its place in the periodic table.
The higher the atomic number, the stronger the electronegativity.

2) The atomic radius
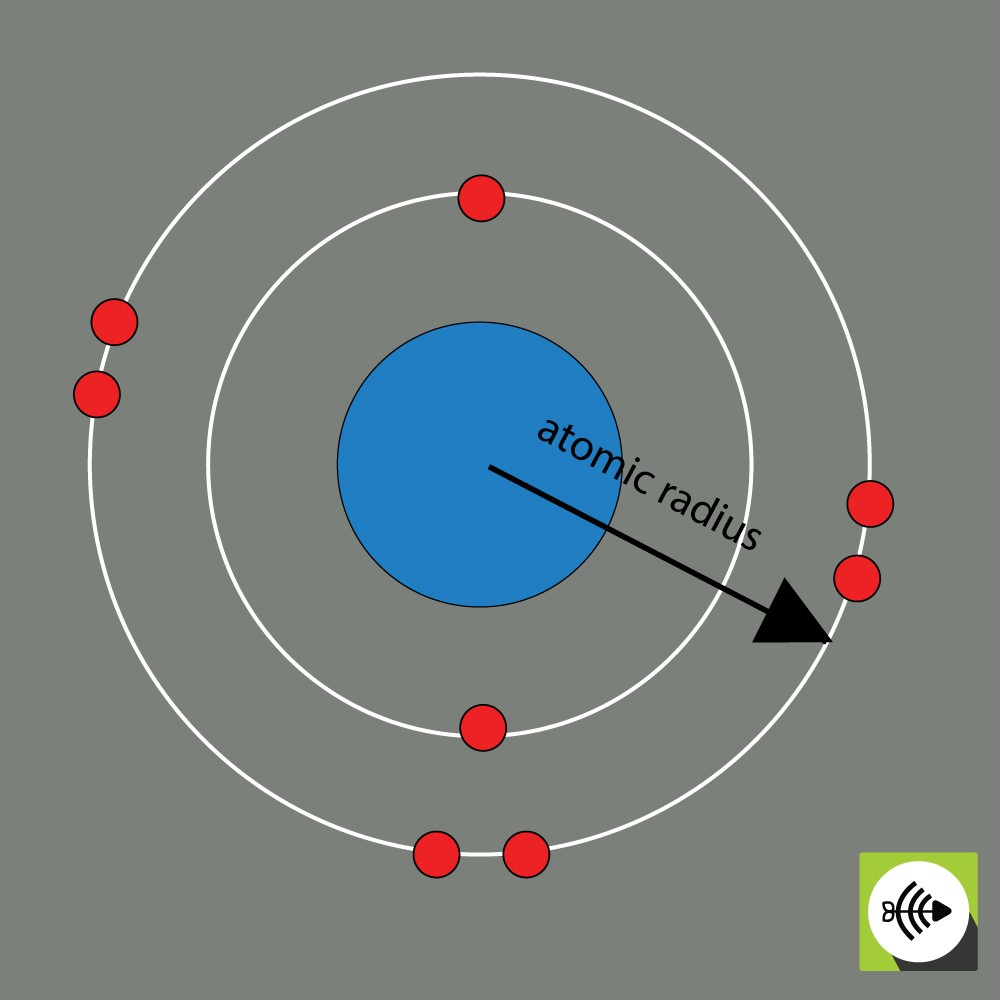
The atomic radius of a chemical element is usually measured by the distance from the center of the nucleus to the boundary of the outermost layer of electrons.
The bigger the atomic radii, the weaker the electronegativity.
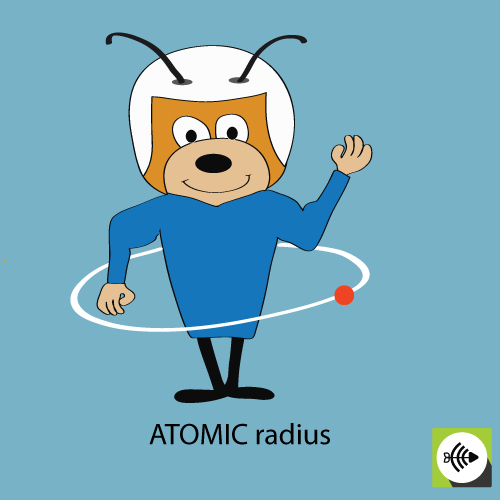
Example of atomic radius of an oxygen atom
What is electronegativity measured in?
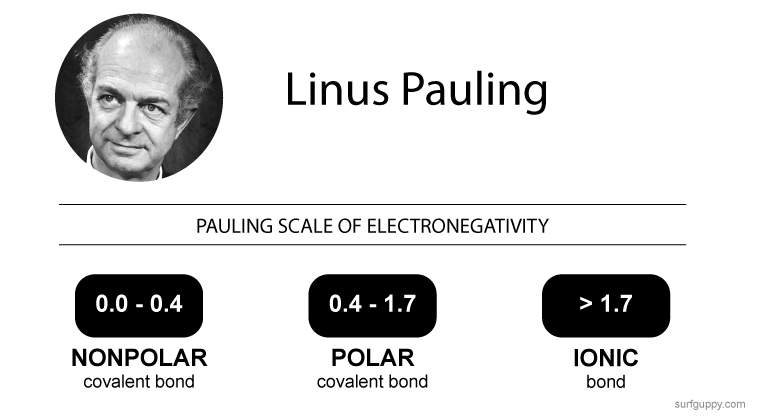
The most common measurement for electronegativity is the Pauling scale, designed by two-time Nobel prize winner Linus Pauling. Electronegativity scale for elements tell how strong each element (relative to each other) can attract the bonding electrons to itself. The higher the electronegativity number, the more the atom will attempt to pull electrons to itself.
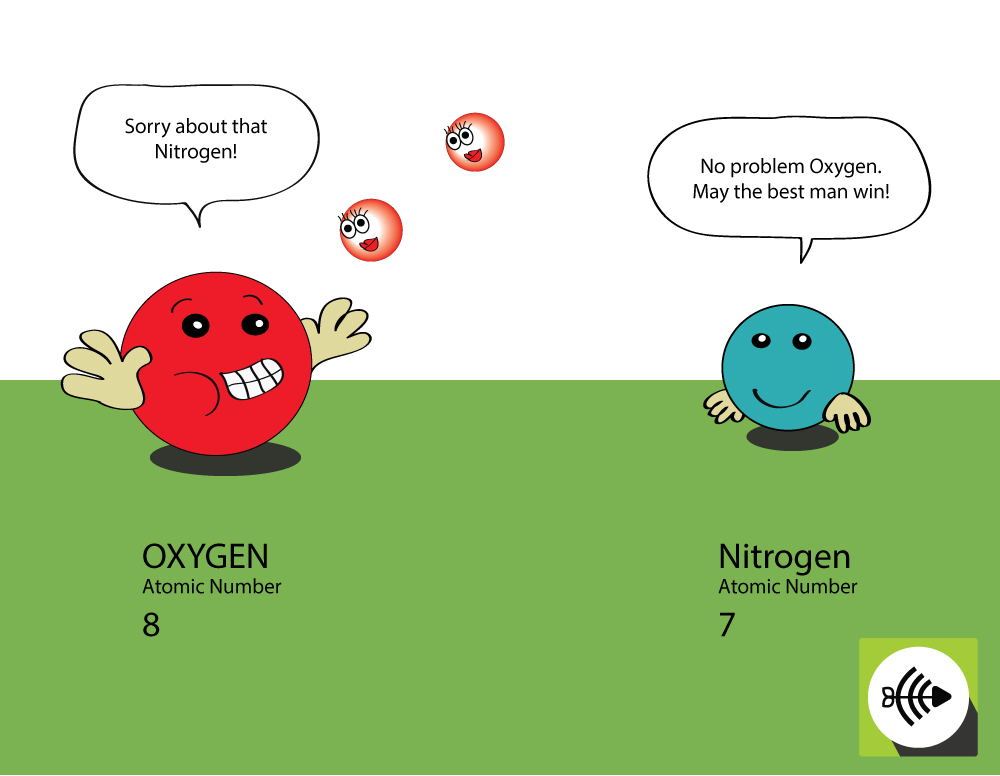
Why is oxygen more electronegative than nitrogen?
Oxygen is higher in atomic number
- Oxygen has 8 protons in the nucleus whereas nitrogen only has 7.
- A bonding pair of electrons will experience more attraction from the oxygen’s nucleus that from nitrogen’s, thus the electronegativity of oxygen is greater.
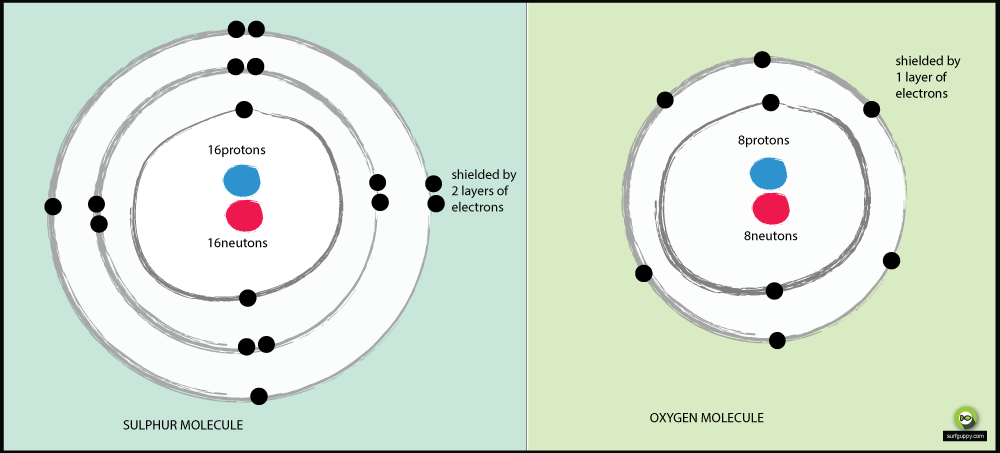
Why is sulphur less electronegative than oxygen?
Reasons why oxygen is more electronegative than sulfur:
- Oxygen has 2 energy levels, sulfur has 3
- The bonding electrons in sulfur are further away from the nucleus of the atom
- When bonding electrons are further from the nucleus of the atom, there is less attraction from the nucleus
- The bonding pair of electrons in oxygen will experience more attraction from its nucleus than sulfur’s bonding electrons
- Hence oxygen is a more electronegative atom
Please read up on valence electrons before you proceed. Valence Electrons
The smaller atom has a higher electronegativity
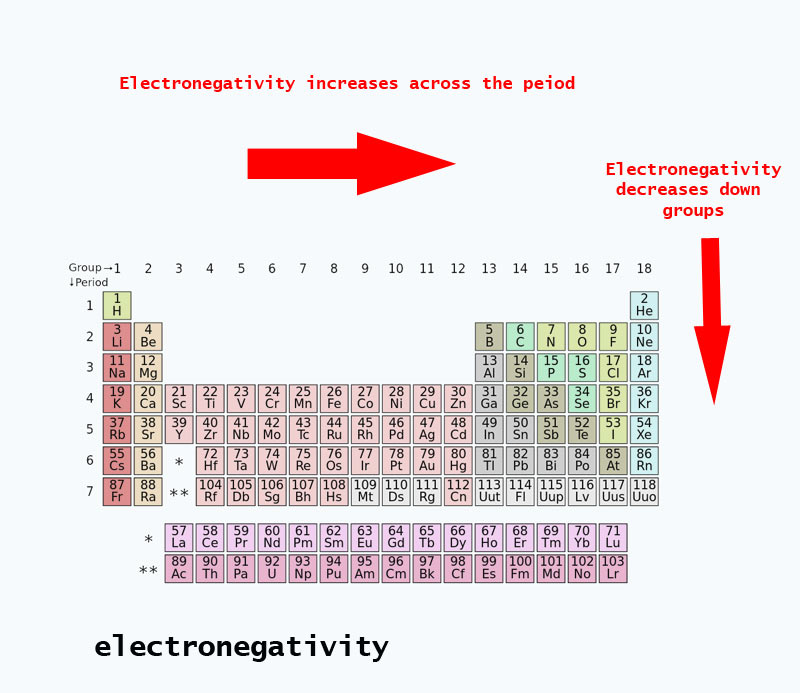
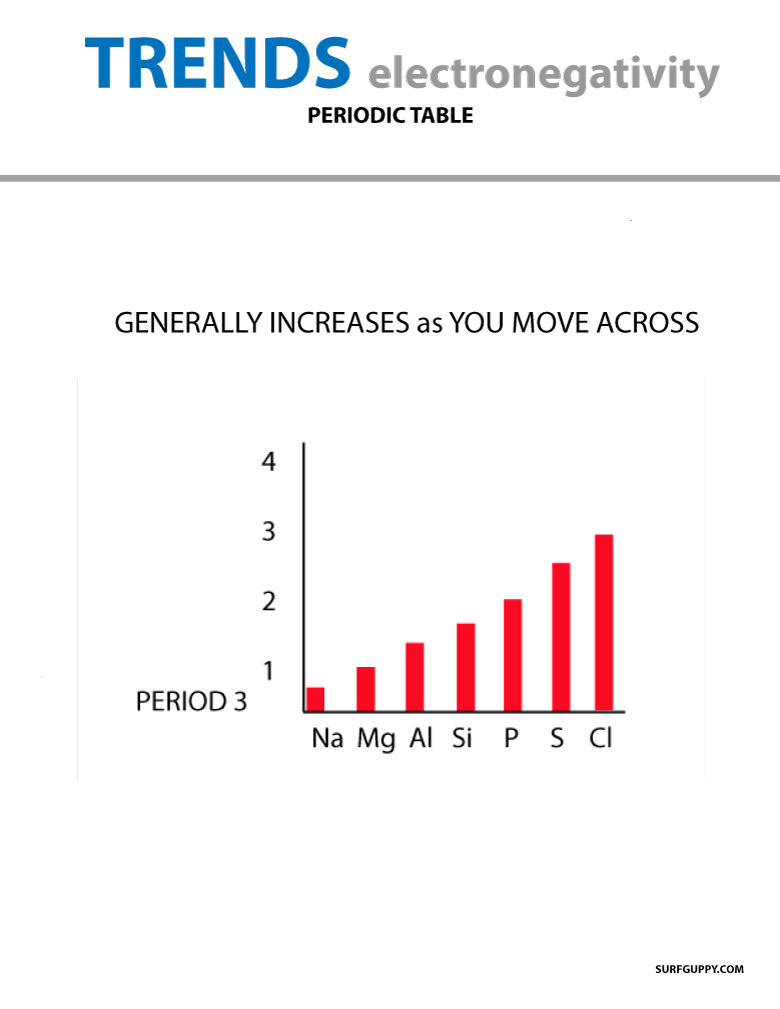
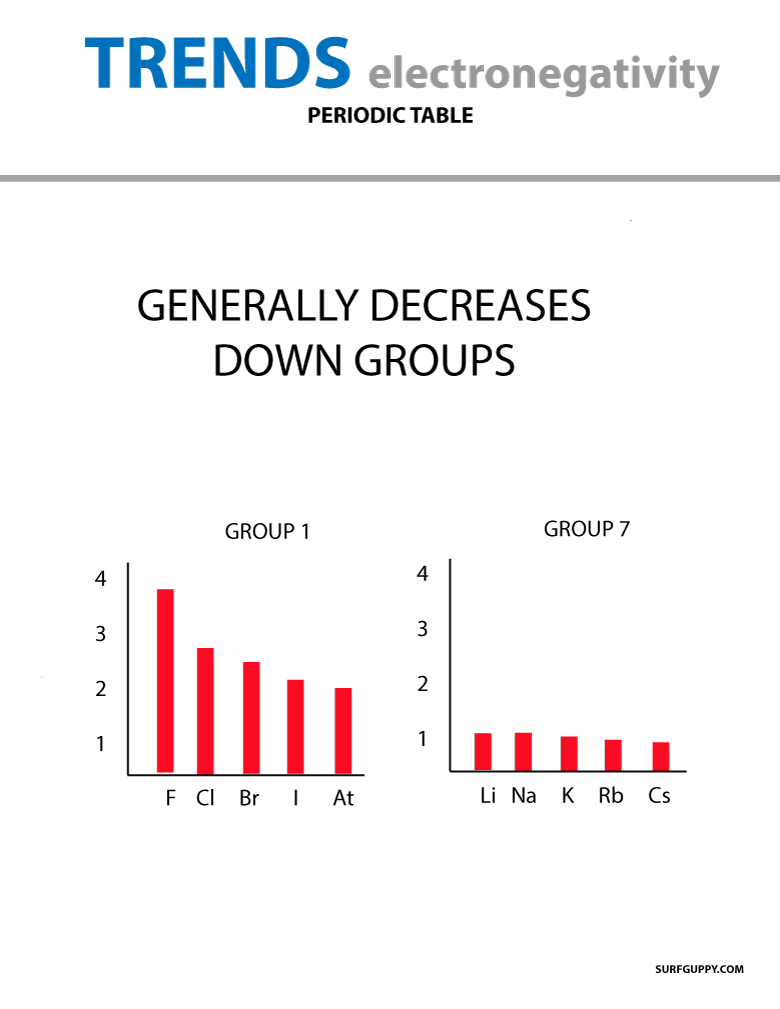
Electronegativity trends present in the periodic table
When you move across the periodic table, the number of protons in the nucleus increases (with no increase in energy level), therefore the electronegativity increases.
When the atomic number increases down a group, there is also an increase in energy levels. The atomic radius is greater therefore electronegativity decreases.
PERIODIC TABLE OF ELECTRONEGATIVITY
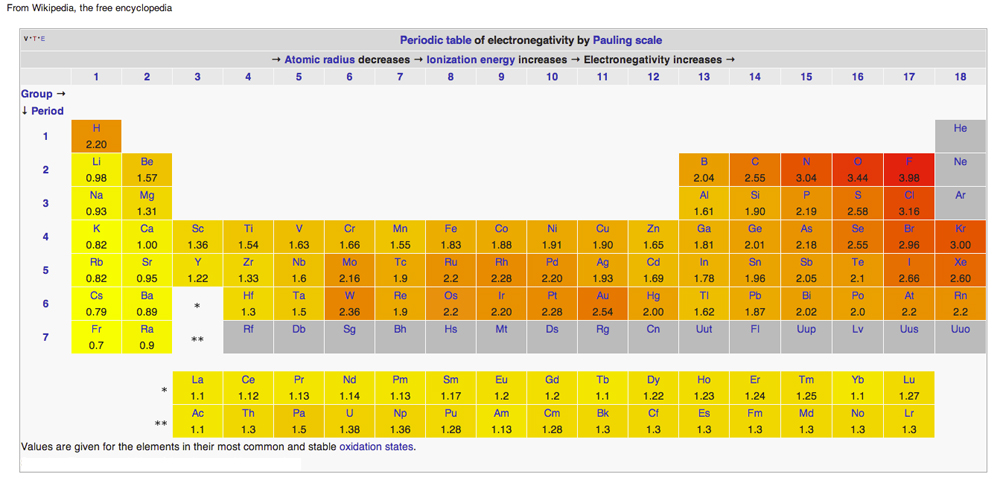 Diagram from Wikipedia issued under Creative Common Licenses
Diagram from Wikipedia issued under Creative Common Licenses