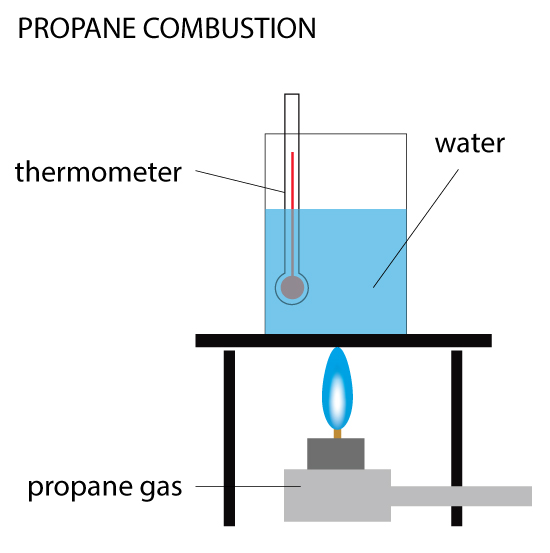
Propane Combustion
When 0.5 g of propane is burned, the heat produced is used to raise the temperature of 100 cm³ of water from 20ºC to 40ºC. Calculate the enthalpy change (ΔH) for the reaction. Given the density of water is 1 g/cm³ and specific heat capacity of water is 4.18 kJ/(kg•C).
In this example, you are calculating the heat change indirectly by measuring the change in temperature of the water. As the methane burns, the heat it expels is absorbed by the water above it. This method isn’t very accurate because some heat is lost to the environment (not all goes to the water) and the loss isn’t recorded. But for a small experiment held in the laboratory, this is an effective method.

Energy transferred
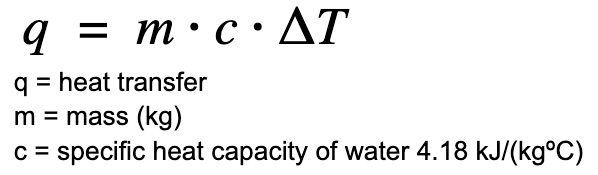
The “•” sign is used interchangeably with the “x” sign to indicate multiplication.
Convert density of water to correct unit
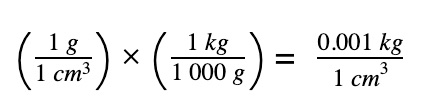
Obtain the mass of water using (density x volume)
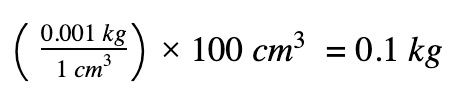
Find temperature change (ΔT)

Find heat transfer (q) to the water
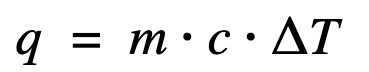
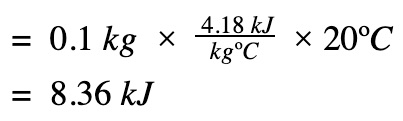
ΔH = q at constant pressure
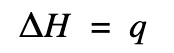
Change the sign
We need to reverse the sign because the heat that is gained by the solution is lost by the reaction
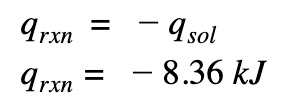
Divide by the number of moles
Moles of propane
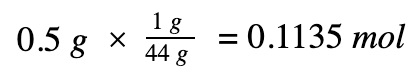
Divide by the number of moles to get molar enthalpy change of the propane gas reaction
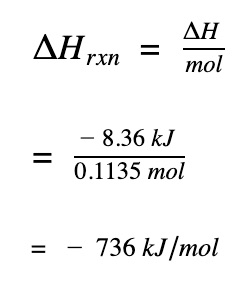
The enthalpy change for the combustion reaction is negative. This indicates the combustion of propane is exothermic and heat is given off. All combustion reactions are exothermic so if you didn’t get a negative value for the ΔH, you
Calculations of enthalpy of a reaction using bond energy and bond enthalpy


0.5 gr * 1 mole / 44 gr = 0.01136 mole
molar enthalpy change of propane
-8.36 kj / 0.01136 = 735.92 kj / mole