| Hi guys! Enthalpy change for methane formation can be really confusing. Here’s a step-by-step explanation to help you see the connections between the processes. Once you’ve understood the concept, write the equations and try it out yourself. |
| For the calculations below, I just want to bring your attention to the hydrogen combustion equation. You might be wondering why I’m using ½ a mole of oxygen. While methane formation equation uses 2 moles of hydrogen, the hydrogen combustion uses ½ mole of oxygen to 1 mole of hydrogen to produce 1 mole of water. This is multiplied by a factor of 2 further down the path as you will see in the diagram. |
| Applying Hess Law, I’m combining different paths to find the enthalpy change of methane formation. You’ll see at the end that you arrive back at the original methane formula even though you have started with other equations. This proves that it doesn’t matter which path you take! |
What is the enthalpy change for the reaction:
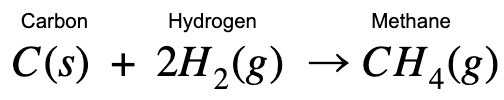
Adjust individual reactions
Carbon combustion
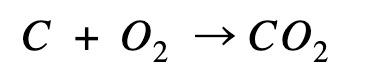
Hydrogen combustion
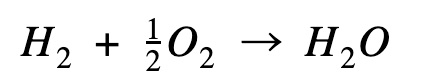
Multiply hydrogen combustion by 2 to get rid of the 1/2 mole
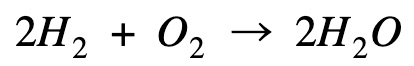
Methane combustion
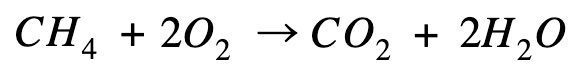
Reverse the equation so that methane is on the product side
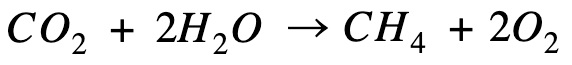
Combine processes
Use the slider below to see how the reactions are combined.
Enthalpy change for the reactions
| Combustion reactions | ∆H (kJ/mol-rxn) | |
| carbon combustion | -293.5 | |
| hydrogen combustion | -285.8 | Multiply by 2 |
| methane formation | -890.3 | Reverse the sign |
(-393,5) + 2(-285.8) + (890.3) = -74.8 kJ/mol-rxn


Thanks… It’s very helpful for me.. I request give more updates to me of your page
as someone doing chem A level, this was very useful!! thank you sir 🙂
yesss!! 100% this was useful
i gotta say that this used a different method to what we were taught in class BUT it was helpful nonetheless
keep doing what you’re doing sir this was very helpful !! you’re helping a load of chem A level students out there
Very Very useful
Hi, I’m confused when you under combine processes, you say methane combustion when the equation written down is for methane formation (CO2 + H2O goes to CH4 and O2)
Thanks for your comment. I’ve corrected the typo and made a slider for it.