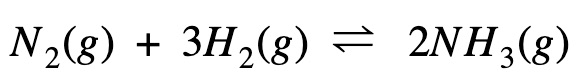Enthalpy Change Calculation
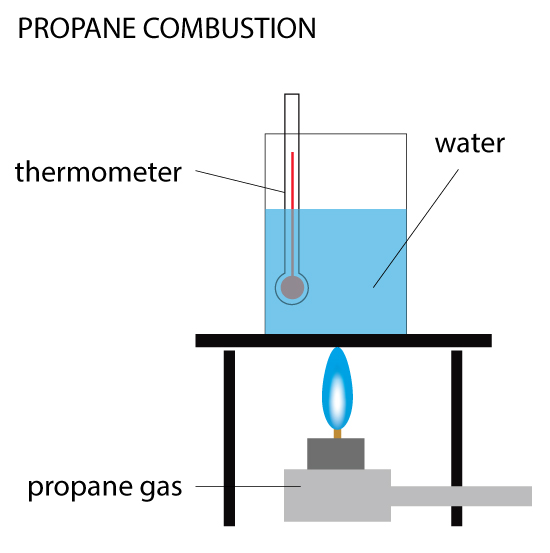
Propane Combustion
When 0.5 g of propane is burned, the heat produced is used to raise the temperature of 100 cm³ of water from 20ºC to 40ºC. Calculate the enthalpy change (ΔH) for the reaction. Given the density of water is 1 g/cm³ and specific heat capacity of water is 4.18 kJ/(kg•C).… Read the rest