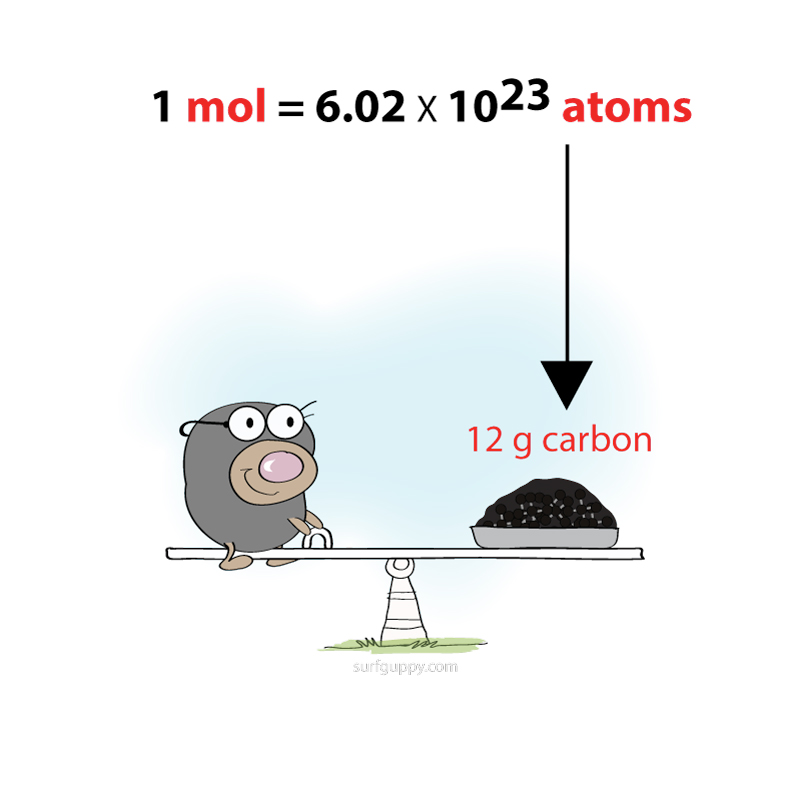
Experiments have determined that in 12 g of carbon there are 6.02 x 10²³ atoms.
1 mole is defined as the number of atoms in 12 g of carbon.
In other words, 1 mole of carbon contains 6.02 x 10²³ atoms!

Experiments have determined that in 12 g of carbon there are 6.02 x 10²³ atoms.
1 mole is defined as the number of atoms in 12 g of carbon.
In other words, 1 mole of carbon contains 6.02 x 10²³ atoms!