
Definition
The valence electrons are the electrons in the outermost shell or last energy level of an atom.
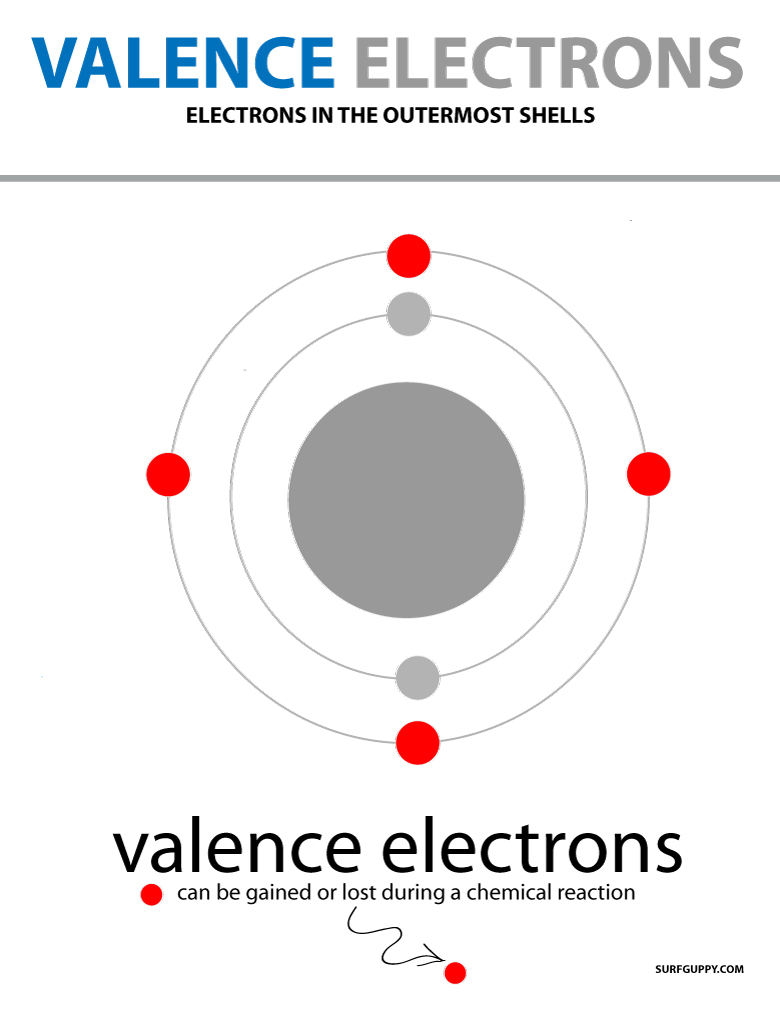
valence electrons are electrons in the last shell of an atom
Valence electrons are electrons in the highest principal energy level.
They are electrons that sit in the outermost shell of an atom.
They are responsible for the chemical properties of each element.
THE SHELLS OF AN ATOM
The maximum number of electrons that can be in the same shell is fixed, and they are filled from the closest to farthest shell
- K Shell (this is the closest shell to the nucleus): 2 electrons maximum
- L Shell: 8 electrons maximum
- M Shell: 18 electrons maximum
- N Shell: 32 electrons maximum
- O Shell: 50 electrons maximum
- P Shell (this is the farthest shell from the nucleus): 72 electrons maximum
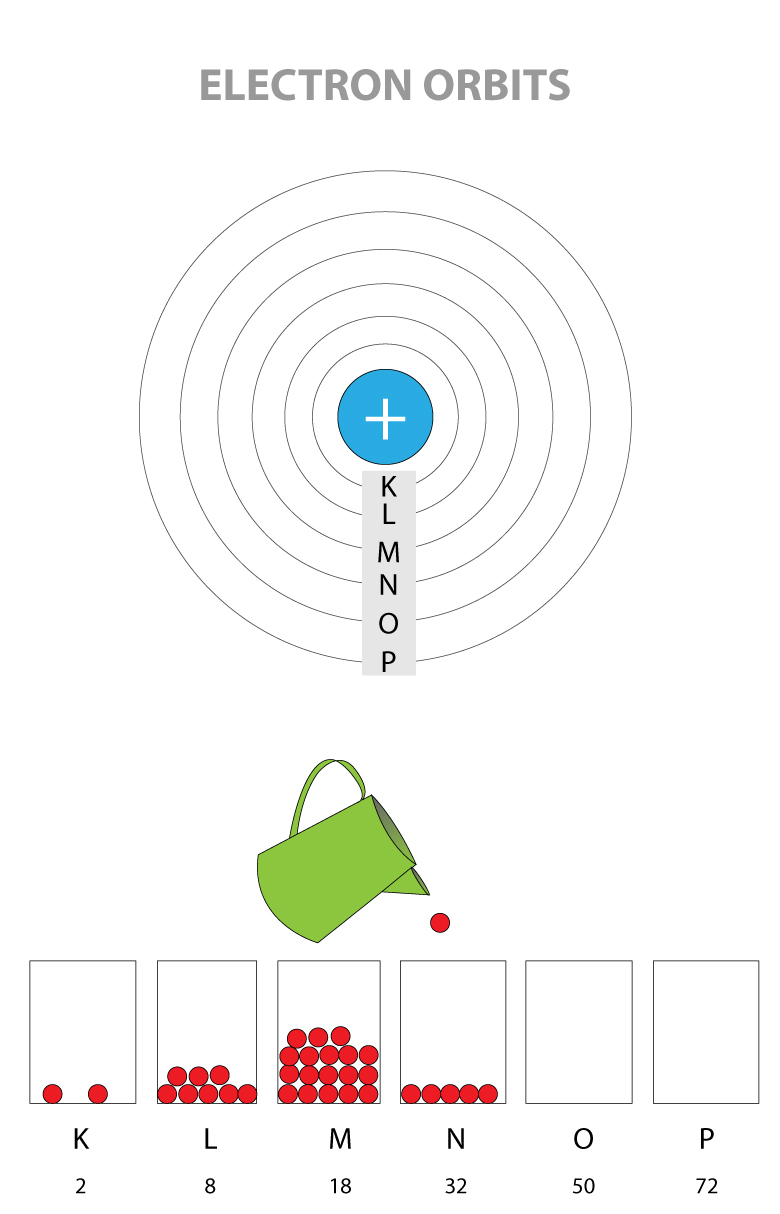
K shell is one closest to the nucleus and has the lowest energy level. Electrons are filled from the lowest energy shell first before going up to higher energy level.
HOW TO FIND THE NUMBER OF VALENCE ELECTRONS FROM A PERIODIC TABLE
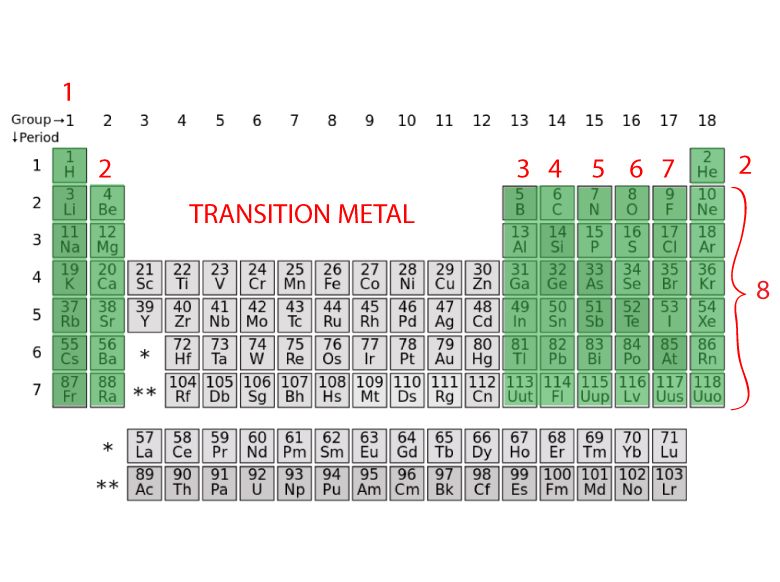
- The Group 1 atoms have 1 valence electron
- The Group 2 atoms have 2 valence electrons
- The Group 13 atoms have 3 valence electrons
- The Group 14 atoms have 4 valence electrons
- The Group 15 atoms have 5 valence electrons
- The Group 16 atoms have 6 valence electrons
- The Group 17 atoms have 7 valence electrons
- The Group 18 atoms have 8 valence electrons with the exception of Helium which has 2 electrons
- The transition metals are more difficult to determine the number of valence electrons. Some of their valence electrons are in the inner shells.
OCTET RULE
The octet rule states that atoms tend to gain, lose, or share electrons so as to have eight electrons in their outer shell because this is more stable.
But the octet rule does not always apply and some bonds that don’t follow the octet rule still form.
For more information on the octet rule of stable electron configuration, please read the octet rule


this was supper help full thank you
The thing about each period filling before going onto the next is wrong. Look at a potassium molecule. It goes onto a 4th period before finishing.